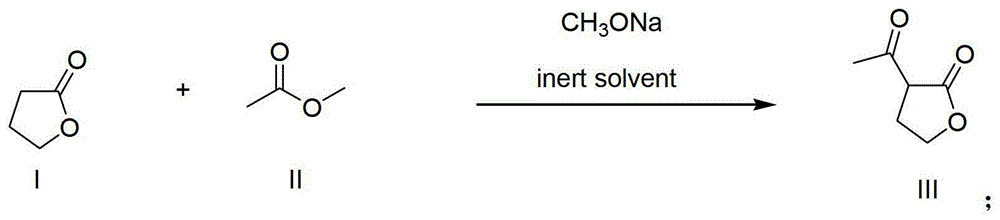Method for preparing alpha-acetyl-gamma-butyrolactone by using recycled reaction material
A technology of butyrolactone and acetyl, which is applied in the field of preparation of α-acetyl-γ-butyrolactone, can solve the problems of difficult removal of high boiling point solvents, high equipment requirements, and low cost, so as to suppress the formation of reaction impurities , Improve yield and purity, reduce the effect of impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
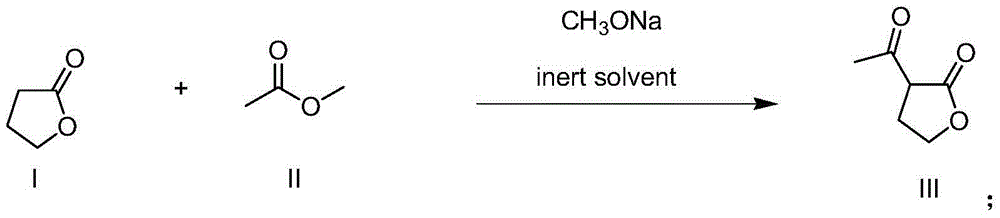
[0037] Example 1 Preparation of α-acetyl-γ-butyrolactone
[0038] Put 6kg (69.8mol) of butyrolactone, 41.2kg (556.6mol) of methyl acetate, 30.9kg (291.5mol) of xylene and 5.3kg (97.4mol) of sodium methoxide in a common reactor and mix and stir slowly. Heat up to 74°C, keep warm and reflux until the gas chromatography detects that the raw material butyrolactone is less than 2%, stop heating, evaporate part of the solvent, adjust the pH value of the solution to 3-4 with 50% phosphoric acid, separate the organic phase, and use the phosphate aqueous phase 10kg of xylene was extracted, the organic phases were combined, and the solvent was evaporated to obtain 10.5kg of α-acetyl-γ-butyrolactone crude product with a content of 74% (crude yield 86.9%), and then obtained α - Pure Acetyl-γ-Butyrolactone.
[0039] Yield: 7.3kg (81.7%);
[0040] Purity: 99.1% (GC).
[0041] Recovery and treatment of mixed waste solvents: After all solvents are combined (about 81.3kg), they are separate...
Embodiment 2
[0042] Example 2 Preparation of α-acetyl-γ-butyrolactone
[0043] The preparation of sodium methylate: the 4.51kg methyl alcohol and the 1.13kg sodium hydroxide that embodiment 1 reclaims gained adopt industrially mature methyl alcohol sodium hydroxide method to prepare sodium methylate, rectification and dehydration after reaction obtain sodium methylate 6.8kg, use as catalyst This example prepares the preparation of α-acetyl-γ-butyrolactone.
[0044] Preparation of α-acetyl-γ-butyrolactone
[0045] With 6kg (69.8mol) butyrolactone, 10.3kg (139.2mol, the raw material is derived from the recovery of Example 1) methyl acetate, 30.9kg (291.5mol, the raw material is from the recovery of Example 1) xylene and 5.3kg ( 98.1mol) sodium methoxide was mixed and stirred in an ordinary reaction kettle, and the temperature was slowly raised to 74°C, and the mixture of methyl acetate and methanol was azeotropically distilled until the reaction temperature reached 85°C, and then 5.2kg (69....
Embodiment 3
[0049] Example 3 Preparation of α-acetyl-γ-butyrolactone
[0050] The preparation of sodium methylate: the 4.78kg methyl alcohol and the 1.20kg sodium hydroxide that embodiment 2 reclaims gained adopt industrial mature methyl alcohol sodium hydroxide method to prepare sodium methylate, rectification and dehydration after reaction obtain sodium methylate 7.2kg, use as catalyst This example prepares the preparation of α-acetyl-γ-butyrolactone.
[0051] Preparation of α-acetyl-γ-butyrolactone:
[0052] With 6kg (69.8mol) butyrolactone, 10.3kg (139.2mol, the raw material is derived from the recovery gained in Example 1 and Example 2) methyl acetate, 30.9kg (291.5mol, derived from the recovered gain in Example 2) xylene and Mix and stir 4.7kg (87.0mol) sodium methoxide in an ordinary reactor, slowly raise the temperature to 74°C, distill out the mixture of methyl acetate and methanol by azeotropic distillation until the reaction temperature reaches 85°C, then add 5.2kg (69.6mol, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com