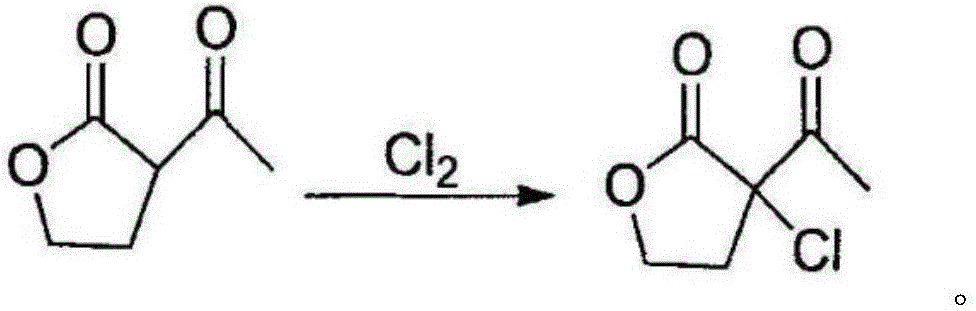
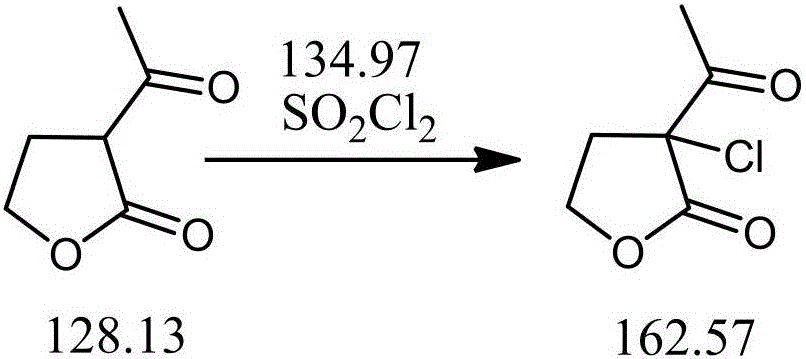Preparation method of alpha-chloro-alpha-acetyl-gamma-butyrolactone
A technology of acetyl and butyrolactone, which is applied in the direction of organic chemistry, can solve the problems of toxic and harmful waste gas, great harm to the human body and the environment, and low yield of reaction, so as to reduce pollution, increase the price and increase the yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 1000mL four-neck round bottom flask equipped with mechanical stirring, thermometer and condenser tube, add 500g of α-acetyl-γ-butyrolactone and lower it to -10°C, then pass chlorine gas at this temperature to keep the chlorine gas React in a saturated state for 5 hours, stop the chlorine gas after the reaction, and change to nitrogen gas to drive away the excess chlorine gas and hydrogen chloride gas generated by the reaction in the system, and then wash with 10% aqueous sodium bicarbonate solution to obtain 641.5g colorless α-chloro- α-acetyl-γ-butyrolactone, content 98.2%, yield 99.3%.
Embodiment 2
[0024] In a 1000mL four-neck round bottom flask equipped with mechanical stirring, thermometer and condenser tube, add 500g of α-acetyl-γ-butyrolactone and lower it to -5°C, then pass chlorine gas at this temperature to keep the chlorine gas React in a saturated state for 5 hours, stop the chlorine gas after the reaction is over, and switch to nitrogen to drive away the excess chlorine and hydrogen chloride gas generated by the reaction in the system, and then wash with 10% aqueous sodium bicarbonate to obtain 644.4g colorless α-chloro- α-acetyl-γ-butyrolactone, content 96.5%, yield 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com