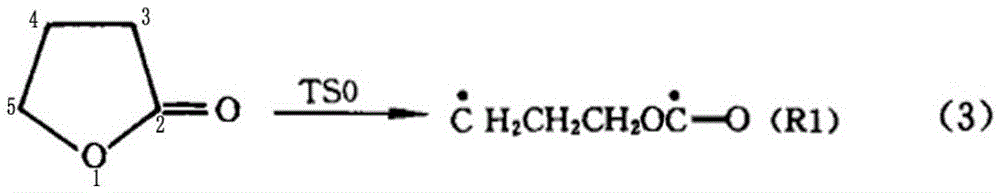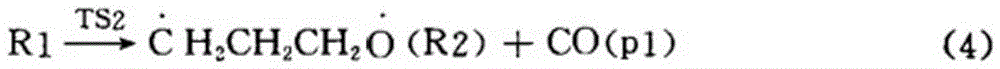Method for preparing cyclopropyl methyl ketone
A technology of cyclopropyl methyl ketone and dimethyl, which is applied in the field of preparation of cyclopropyl methyl ketone, can solve the problems of a large amount of waste acid, water and solid waste residues, excessive amounts of catalysts and solvents, and serious equipment corrosion. Achieve high production efficiency, improve selectivity and conversion rate, and reduce environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
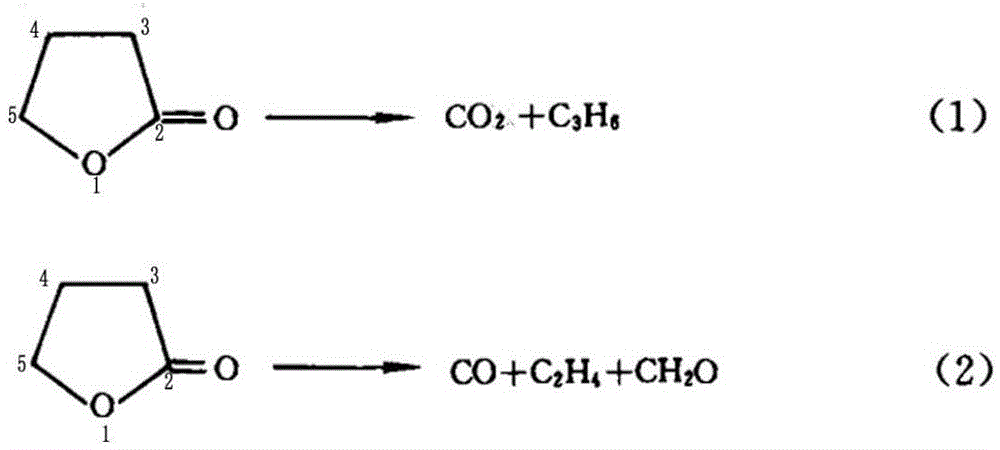
[0049] A preparation method of cyclopropyl methyl ketone, said preparation method comprising the steps of: adding metal halides and inert solvents as described in Table 1, Example 1, to a three-stage series fixed stage with a reaction pressure of 0.6MPa In the fixed-bed reactor, the fixed-bed reactor is heated to 190°C, and then α-acetyl-γ-butyrolactone is continuously added to the fixed-bed reactor to cause the cleavage reaction of α-acetyl-γ-butyrolactone;
[0050] When the reaction is saturated, stop adding α-acetyl-γ-butyrolactone, and continue the reaction distillation until no product is evaporated to obtain the crude product of cyclopropyl methyl ketone. After the distillation of the product is completed, slowly open the vacuum to recover the inert solvent and sodium iodide;
[0051] The fixed-bed reactor is connected with a rectification tower, and the crude product of cyclopropyl methyl ketone is transferred to a three-way rectification tower, and the high tower is fir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com