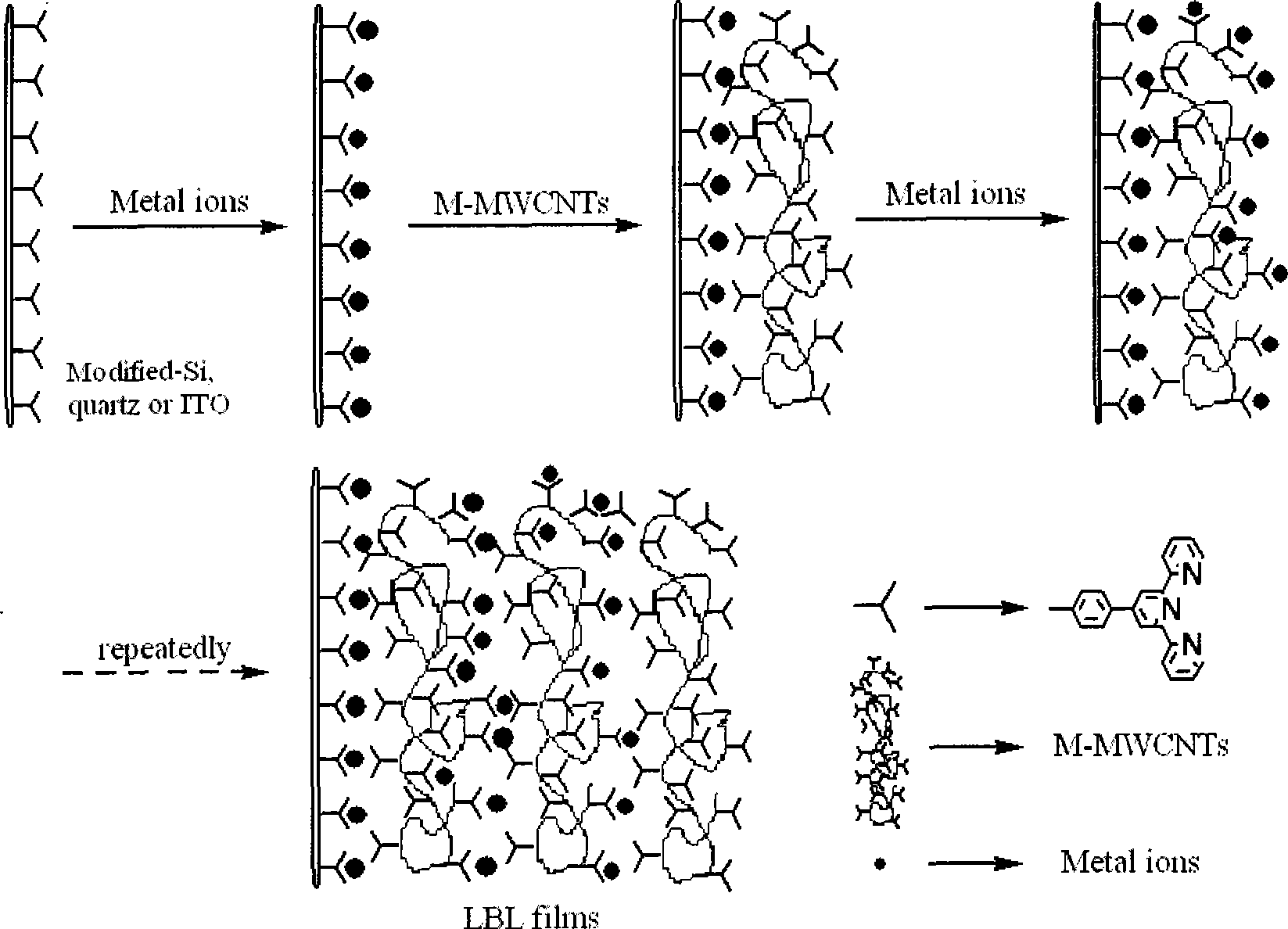
Coordination layer-by-layer self-assembly of terpyridyl covalent modified multi-wall carbon nanotube and transition metal ion on functional substrate surface
A technology of multi-walled carbon nanotubes and transition metal ions, applied in the fields of functional materials, supramolecular self-assembly chemistry, and nanomaterials, can solve the problem that the stability is easily affected by the environment, reduce the optical or electrical properties, and the terpyridine group and the The surface layer of carbon nanotubes has no problems such as being able to achieve conjugation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
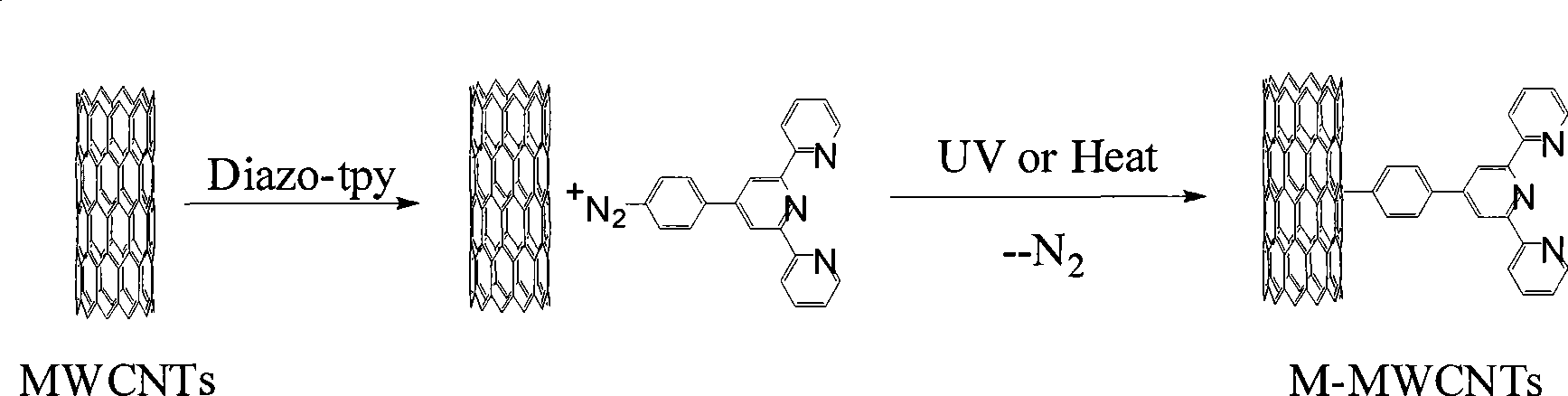
[0027] In the first step, in a 100ml round bottom flask, dissolve 10mg of pure multi-walled carbon nanotubes in 50ml of acetonitrile, and disperse them fully by ultrasonication for 30min. liquid.
[0028] In the second step, under stirring, add the suspension of MWCNTs ultrasonically treated in the first step into 50 ml of Diazo-tpy / acetonitrile solution with a concentration of 0.2 mg / ml using a dropper. The reaction was stirred at 0°C and protected from light for 48 hours, so that Diazo-tpy was adsorbed on the sidewall surface of MWCNTs through electrostatic interaction.
[0029] The third step, after the reaction is over, tear off the tinfoil covering the round-bottom flask, quickly expose it to a 400W ultraviolet lamp, and irradiate it for 30 minutes while stirring. Through the photoreaction of the diazo group, the loss of nitrogen produces crosslinking. The terpyridine group is directly connected to the outer wall of MWCNTs in the form of a covalent bond (the modificatio...
Embodiment 2
[0038] In the first step, in a 100ml round bottom flask, dissolve 10mg of pure multi-walled carbon nanotubes in 50ml of acetonitrile, and disperse them fully by ultrasonication for 30min. liquid.
[0039] In the second step, under stirring, add the suspension of MWCNTs sonicated in the first step into 50 ml of Diazo-tpy / acetonitrile solution with a concentration of 0.4 mg / ml using a dropper. The reaction was stirred for 48 hours at 0° C. and protected from light.
[0040] The third step, after the reaction is over, tear off the tinfoil covering the round-bottom flask, quickly expose it to a 400W ultraviolet lamp, and irradiate it for 30 minutes while stirring. Through the photoreaction of the diazo group, the loss of nitrogen produces crosslinking. The terpyridine group is directly connected to the outer wall of MWCNTs in the form of covalent bonds.
[0041] The fourth step, the post-processing is the same as the fourth step in Example 1.
[0042] The fifth step is the con...
Embodiment 3
[0047] In the first step, in a 100ml round bottom flask, dissolve 10mg of pure multi-walled carbon nanotubes in 50ml of acetonitrile, and disperse them fully by ultrasonication for 30min. liquid.
[0048] In the second step, under stirring, add the suspension of MWCNTs ultrasonically treated in the first step into 50 ml of Diazo-tpy / acetonitrile solution with a concentration of 0.2 mg / ml using a dropper. The reaction was stirred at 0°C and protected from light for 48 hours, so that Diazo-tpy was adsorbed on the sidewall surface of MWCNTs through electrostatic interaction.
[0049] The third step, after the reaction is over, tear off the tinfoil covering the round-bottom flask, quickly expose it to a 400W ultraviolet lamp, and irradiate it for 30 minutes while stirring. Through the photoreaction of the diazo group, the loss of nitrogen produces crosslinking. The terpyridine group is directly connected to the outer wall of MWCNTs in the form of covalent bonds.
[0050] In the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com