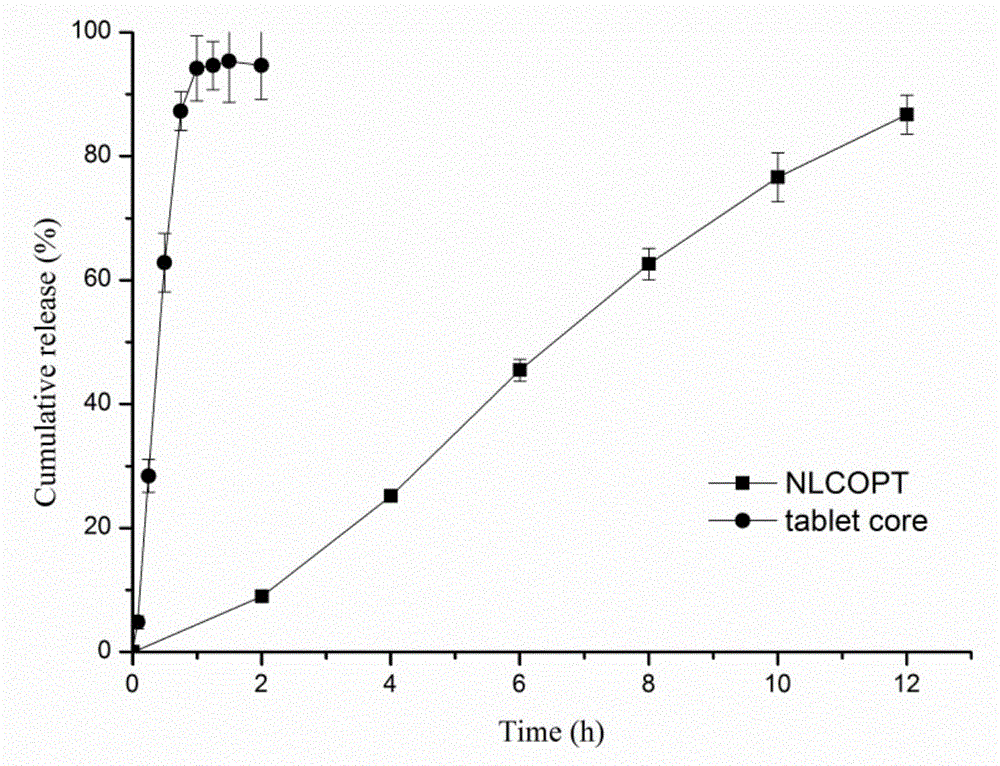
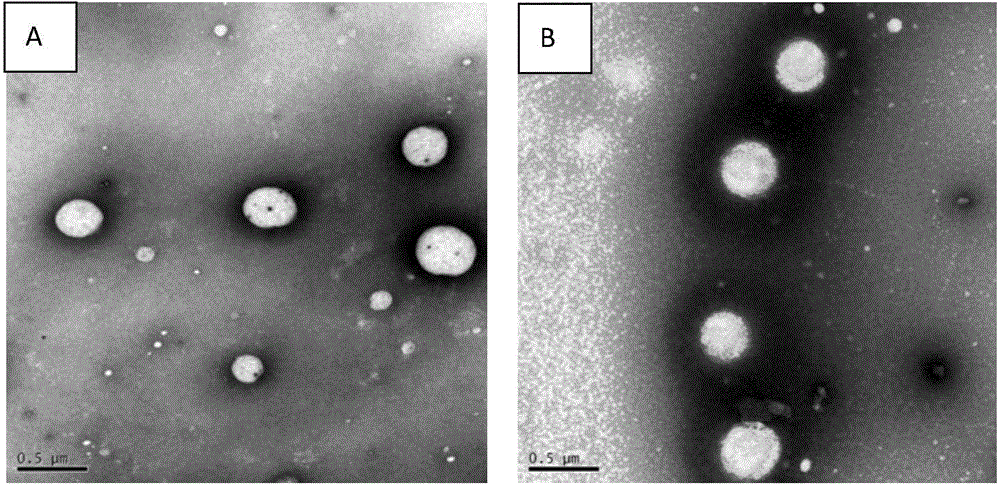
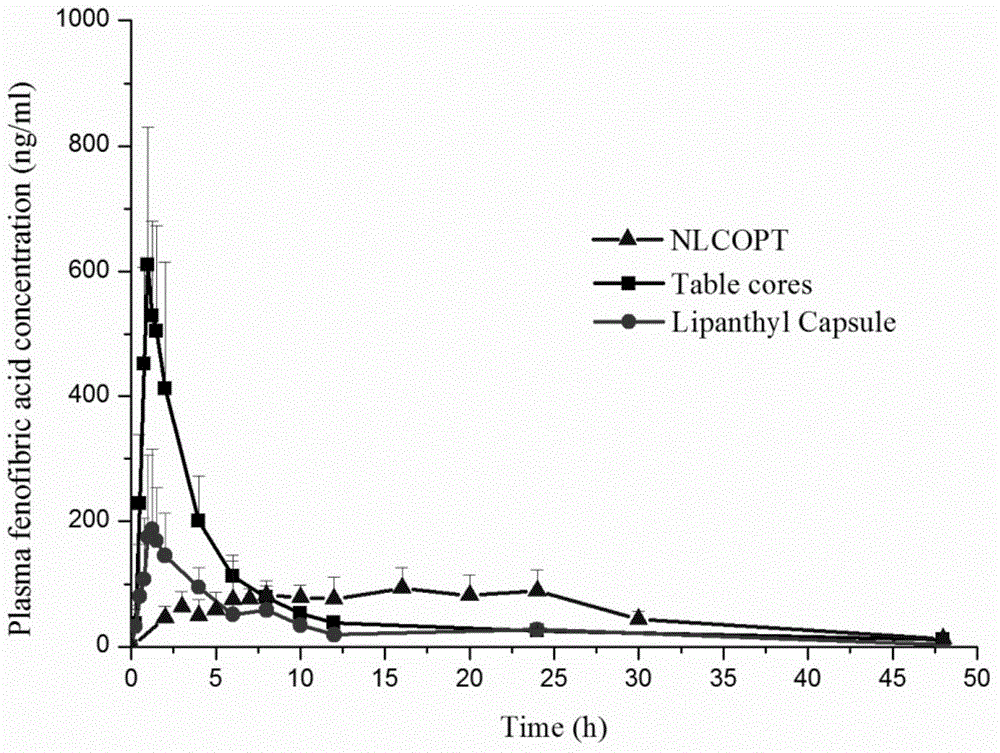
Osmotic pump controlled release preparation capable of controlling 'nano-particle' overall release and preparation method thereof
An osmotic pump controlled release and overall release technology, which is used in the osmotic pump controlled release preparations and their preparations that control the overall release of "nanoparticles", and the field of drug delivery systems that control the overall release of "nanoparticles", which can solve the problem of drug release control. It is very stable, affects drug absorption, affects the size and other issues, and achieves the effects of improving bioavailability, increasing effective blood concentration, and slowing down absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This example provides lipid nanoparticles containing insoluble drug fenofibrate composed of fenofibrate, PrecirolATO5 and LabrafacCC: fenofibrate 3.2g
[0037] PrecirolATO5 38g
[0038] Labrafac CC 16g
[0039] Prepared by the following method:
[0040] 1. Melt and mix the prescribed amounts of fenofibrate, PrecirolATO5 and Labrafac CC in a water bath at 80°C; 2. Pour the oil phase into the water phase containing 2% Tween80 at 80°C under high shear conditions , and stir into colostrum; 3. Quickly transfer the prepared colostrum to a microjet high-pressure homogenizer, circulate and homogenize 3 times, and cool to room temperature to obtain a nanostructure lipid carrier suspension;
[0041] The average particle size of the prepared solid lipid nanoparticles was 123 nm, and the drug encapsulation efficiency was 98.7%.
Embodiment 2
[0043] The present embodiment provides that the self-microemulsion containing insoluble drug cyclosporine is composed of LabrafilM1944CS, Cremophor EL, and TranscutolP:
[0044]
[0045] Prepared by the following method:
[0046] Dissolve cyclosporine A in LabrafilM1944CS, CremophorEL, TranscutolP of recipe quantity and get final product;
[0047] The prepared self-microemulsion can be rapidly dispersed in water as microemulsion droplets of about 20 nm.
Embodiment 3
[0049] This example provides PLGA nanoparticles containing insoluble drug cyclosporine, which is composed of PLGA and PVA:
[0050] Cyclosporine A 3g
[0051] PLGA 15g
[0052] PVA 4.5g
[0053] Prepared by the following method:
[0054] Prepared by emulsification solvent evaporation method. Dissolve cyclosporine A and PLGA in ethyl acetate to form an oil phase, add the oil phase dropwise to a 1.5% PVA aqueous solution, and continue to stir for 1 hour. The particle size is reduced by high pressure homogenization, and finally the emulsion is poured into a 1.5% PVA aqueous solution, and the ethyl acetate is completely volatilized to obtain PLGA nanoparticles;
[0055] The prepared PLGA nanoparticles have a round appearance and a particle size of about 182.2 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com