Polymer mixtures of anionic and cationic polysaccharides anduse thereof
A technology for anionic polysaccharides and mixtures, which is applied in the fields of drug combination, medical science, bone diseases, etc., can solve the problems of no mixture, no coexistence of anionic and cationic polymers, loss and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
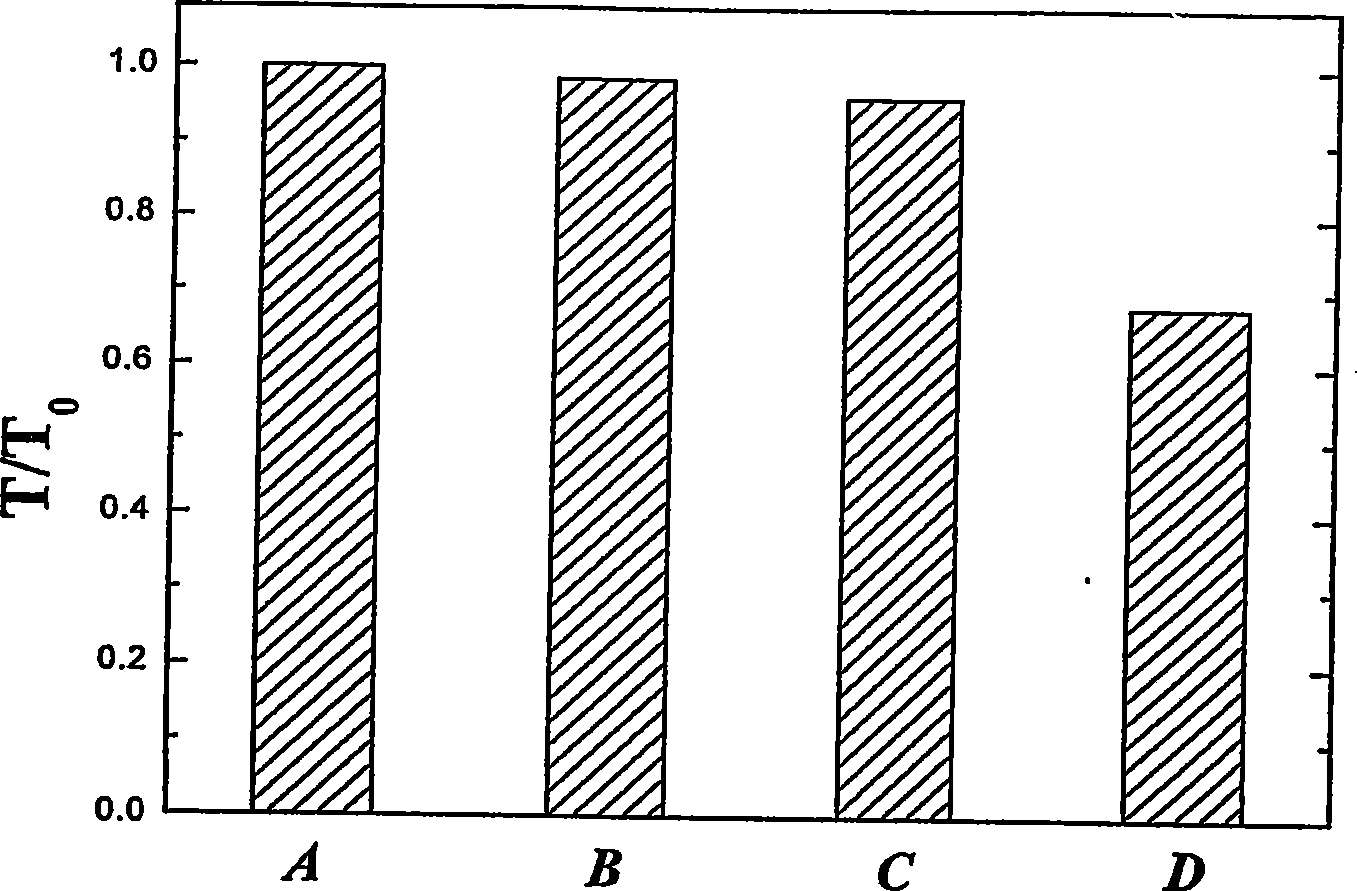
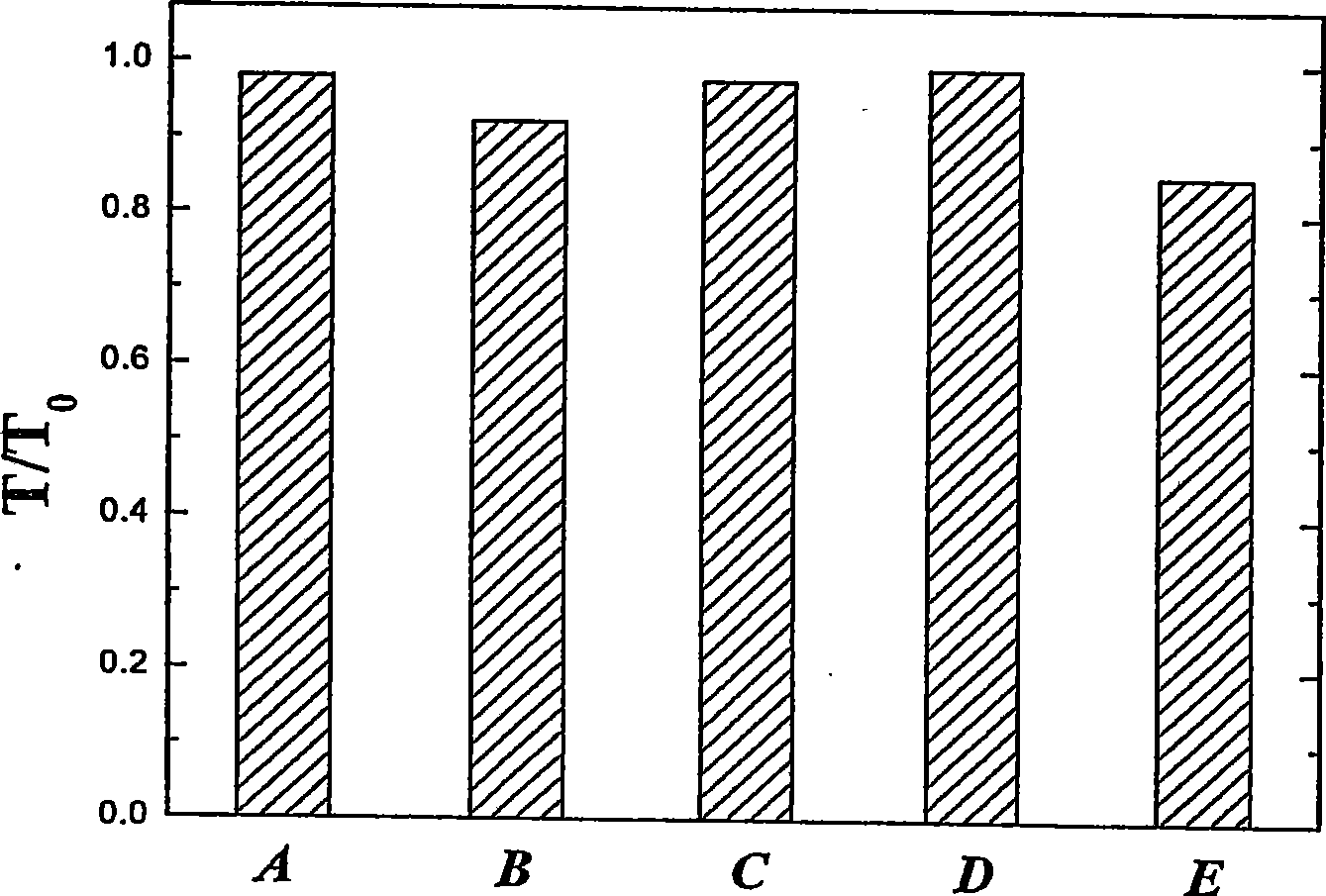
Image
Examples
example 1
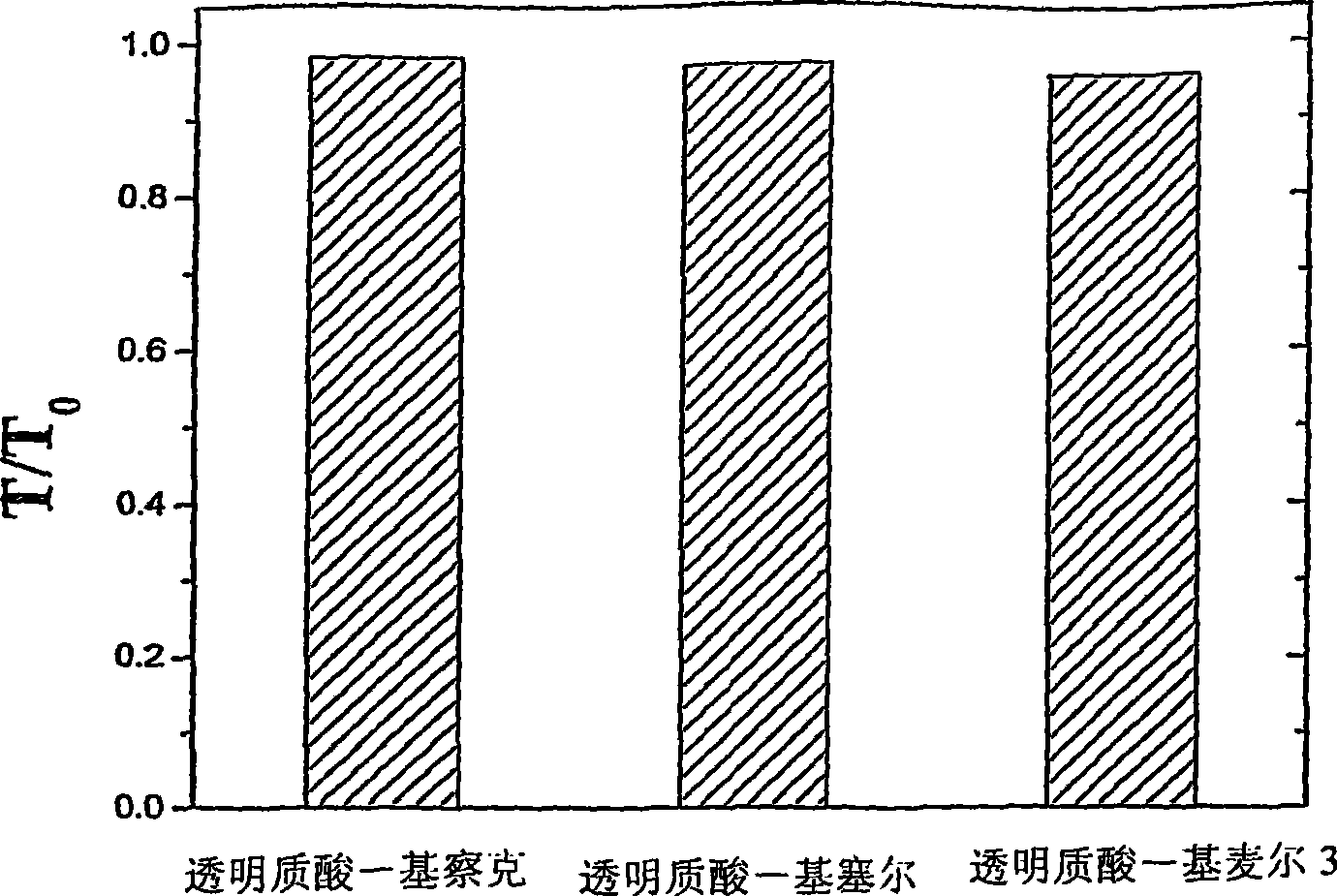
[0047] example 1: Synthetic Chitosan Lactose Derivatives (known as Kitrak)
[0048] Chitosan (1.5 g, degree of acetylation 11%) was dissolved in 110 mL of methanol (55 mL) and 1% acetic acid buffer (pH 4.5 (55 mL)) solution. 60 mL of a solution of lactose (2.2 g) and sodium cyanoborohydride (900 mg) in methanol (30 mL) and 1% acetic acid buffer (pH 4.5 (30 mL)) was added. The mixture was stirred for 24 hours, transferred to a dialysis tube (cutoff: 12000 Da) and dialyzed against 0.1 M NaCl (2 times) and deionized water until the conductivity was 4 μS (4° C.). Finally the solution was filtered through a microporous (0.45 μm) filter and lyophilized.
example 2
[0049] Example 2: Synthesis of Chitosan Cellobiose Derivatives (hereinafter referred to as Kissel)
[0050] Chitosan (1.5 g, degree of acetylation 11%) was dissolved in 110 mL of methanol (55 mL) and 1% acetic acid buffer (pH 4.5 (55 mL)). 60 mL of methanol (30 mL), 1% acetate buffer (pH 4.5 (30 mL)) solution containing cellobiose (2.2 g) and sodium cyanoborohydride (900 mg) was added. The mixture was stirred for 24 hours, transferred to a dialysis tube (cutoff: 12000 Da) and dialyzed against NaCl 0.1M (2 times) and deionized water until the conductivity was 4 μS (4°C). Finally the solution was filtered through a millipore filter (0.45 μm) and freeze-dried.
example 3
[0051] Example 3: Synthesis of Chitosan Maltotriose Derivatives (hereinafter referred to as Kimmel 3)
[0052] Chitosan (300 mg, degree of acetylation 11%) was dissolved in 22 mL of methanol (11 mL) and 1% acetic acid buffer (pH 4.5 (11 mL)). 12 mL of methanol (6 mL), 1% acetate buffer (pH 4.5 (6 mL)) containing maltotriose (650 mg) and sodium cyanoborohydride (180 mg) was added. The mixture was stirred for 24 hours, transferred to a dialysis tube (cutoff: 12000 Da) and dialyzed against NaCl 0.1M (2 times) and deionized water until the conductivity was 4 μS (4°C). Finally the solution was filtered through a millipore filter (0.45 μm) and freeze-dried.
[0053] The following examples of polysaccharide mixtures are obtained from the aforementioned chitosan derivatives and commercially available polyanionic polysaccharides with different average molecular weights.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com