Liquid crystal oriented agent and liquid crystal display element
A technology of liquid crystal display element and liquid crystal aligning agent, which is applied in the direction of liquid crystal materials, chemical instruments and methods, optics, etc., and can solve the problems of not very good performance, insufficient improvement of afterimage performance, and insufficient voltage retention rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
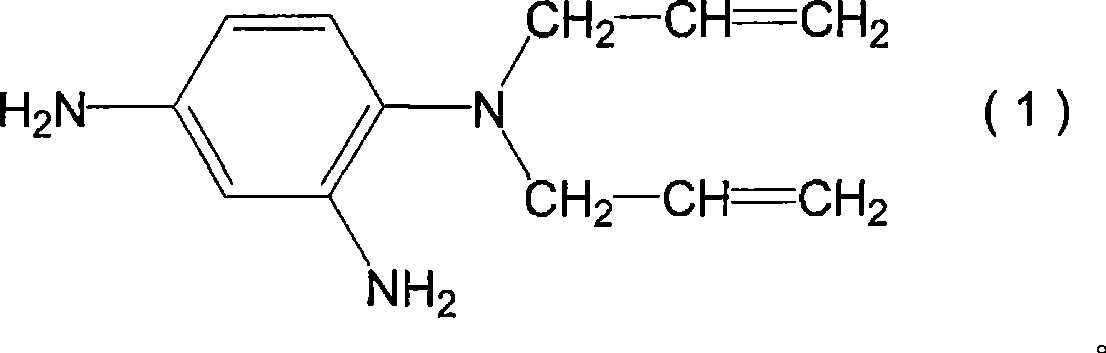
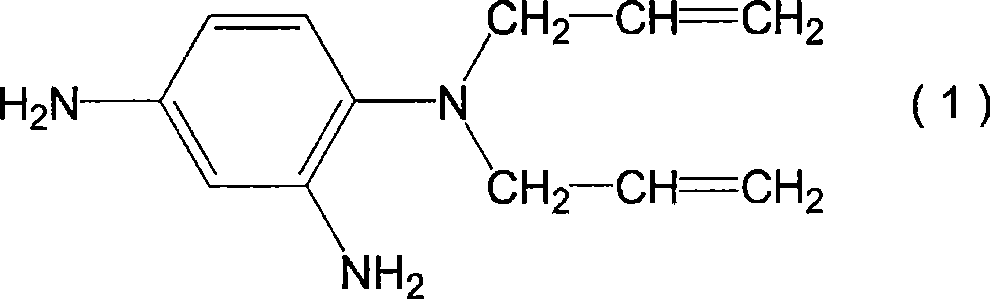
[0125] 93 g (0.48 mol) of 1,2,3,4-cyclobutanetetracarboxylic dianhydride as tetracarboxylic dianhydride and 5 g (0.03 mol) of pyromellitic dianhydride, and 2 as diamine compound, 4-diamino-N, N-diallylaniline (the compound represented by the above-mentioned formula (1)) 81g (0.4 mol) and 52g (0.1 mol) of the compound represented by the above-mentioned formula (D-3) were dissolved in 928g N- Methyl-2-pyrrolidone was reacted at 60° C. for 6 hours to obtain a solution containing polyamic acid. A small amount of the obtained polyamic acid solution was taken, and N-methyl-2-pyrrolidone was added to prepare a solution with a polyamic acid concentration of 10% by weight. The viscosity of the solution was measured to be 53 mPa·s.
[0126] Then, 1160 g of N-methyl-2-pyrrolidone was added to the obtained polyamic acid solution, and 198 g of pyridine and 204 g of acetic anhydride were added, and a dehydration ring-closure reaction was performed at 110° C. for 4 hours. After the dehydrat...
Synthetic example 2
[0128] 93 g (0.48 mol) of 1,2,3,4-cyclobutanetetracarboxylic dianhydride as tetracarboxylic dianhydride and 5 g (0.03 mol) of pyromellitic dianhydride, and 2 as diamine compound, 4-diamino-N, N-diallylaniline 61g (0.3 moles) and 4-[4-(4-trans n-heptylcyclohexyl) phenoxy]-1,3-diaminobenzene 76g ( 0.2 mol) was dissolved in 944g of N-methyl-2-pyrrolidone and reacted at 60°C for 6 hours to obtain a solution containing polyamic acid. A small amount of the obtained polyamic acid solution was taken, and N-methyl-2-pyrrolidone was added to prepare a solution with a polyamic acid concentration of 10% by weight. The viscosity of the solution was measured to be 56 mPa·s.
[0129] Then, 1180 g of N-methyl-2-pyrrolidone was added to the obtained polyamic acid solution, and 198 g of pyridine and 204 g of acetic anhydride were added, and a dehydration ring-closure reaction was performed at 110° C. for 4 hours. After the dehydration ring-closure reaction, by replacing the solvent in the syst...
Synthetic example 3
[0131] 1,2,3,4-cyclobutanetetracarboxylic dianhydride 64g (0.33 mol), 2,3,5-tricarboxycyclopentyl acetic dianhydride 34g (0.15 mol) and 5 g (0.03 mol) of pyromellitic dianhydride, 81 g (0.4 mol) of 2,4-diamino-N,N-diallylaniline as a diamine compound, and the compound represented by the above formula (D-3) 52 g (0.1 mol) was dissolved in 956 g of N-methyl-2-pyrrolidone, and reacted at 60° C. for 6 hours to obtain a solution containing polyamic acid. A small amount of the obtained polyamic acid solution was taken, and N-methyl-2-pyrrolidone was added to form a solution with a polyamic acid concentration of 10% by weight. The viscosity of the solution was measured to be 52 mPa·s.
[0132] Then, 1195 g of N-methyl-2-pyrrolidone was added to the obtained polyamic acid solution, and 198 g of pyridine and 204 g of acetic anhydride were added, and a dehydration ring-closure reaction was performed at 110° C. for 4 hours. After the dehydration ring-closure reaction, by replacing the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com