Disposable levofloxacin hydrochloride eye drops without bacteriostatic agent and preparation method thereof
A technology of ciprofloxacin hydrochloride and eye drops, which can be applied to medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulations, etc., can solve problems such as high risk, save costs and ensure sterility requirements , avoid potentially dangerous effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
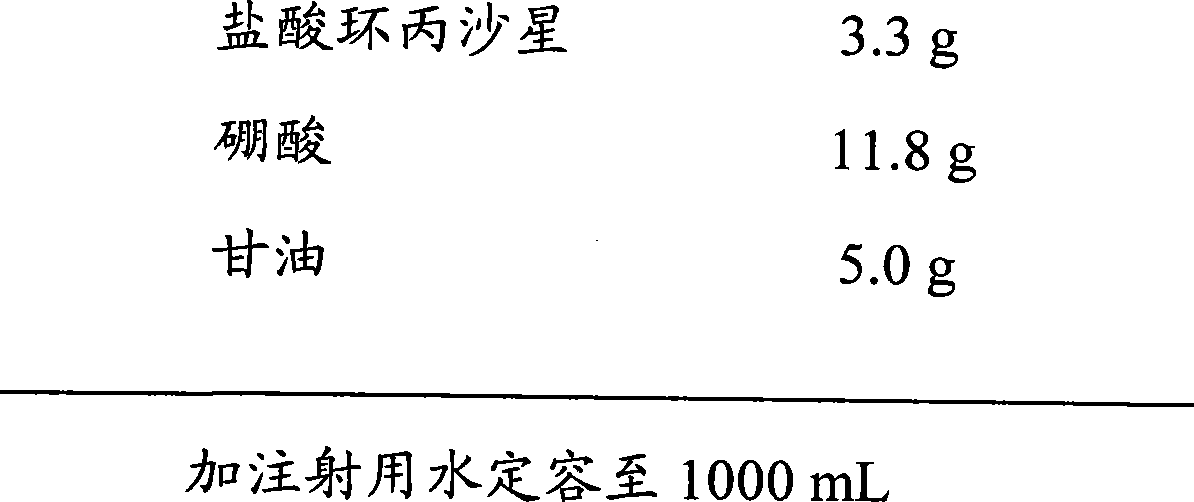
[0045] prescription:
[0046]
[0047] Its preparation method:
[0048] a. Accurately weigh 3.3g of ciprofloxacin hydrochloride, 11.8g of boric acid, and 5.0g of glycerin, add 200mL of water for injection to dissolve, stir evenly, and dilute to 1000mL with water for injection to obtain a medicinal solution.
[0049] b. The above medicinal solution is filtered once through a 0.45 μm microporous membrane, and then filtered twice through a 0.22 μm microporous membrane under a Class 100 environment.
[0050] c. After the medicinal liquid is tested and passed, fill 0.4mL of the above medicinal liquid into a 1mL single-dose packaging plastic container in a class 100 environment, and seal it to obtain the finished product.
Embodiment 2
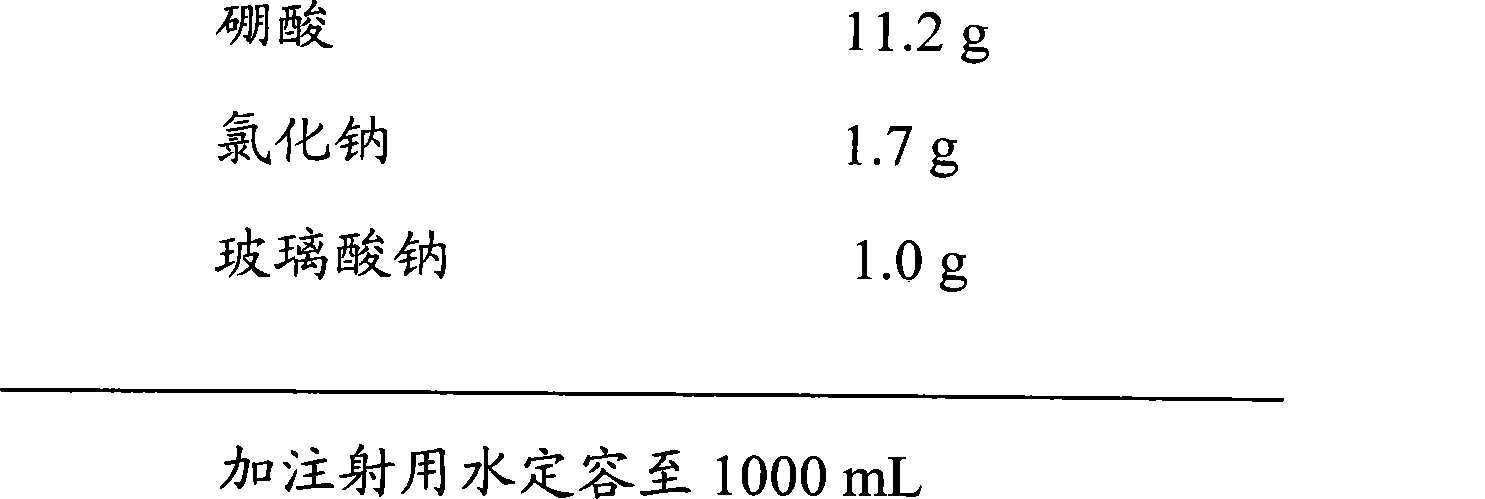
[0052] prescription:
[0053]
[0054]
[0055] Its preparation method:
[0056]a. Accurately weigh 3.3g of ciprofloxacin hydrochloride, 11.2g of boric acid, 1.7g of sodium chloride, and 1.0g of sodium hyaluronate, add 200mL of water for injection to dissolve, stir evenly, and dilute to 1000mL with water for injection to obtain a medicinal solution .
[0057] b. Filter the medicinal solution prepared above through a 0.45 μm microporous membrane once, and then through a 0.22 μm microporous membrane once.
[0058] c. The filtered medicinal solution is sterilized at 121° C. for 8 minutes by autoclaving, and then cooled.
[0059] d. After the medicinal liquid is tested and passed, fill 0.5mL of the above medicinal liquid into a 1mL single-dose packaging plastic container in a class 100 environment, and seal it to obtain the finished product.
Embodiment 3
[0061] prescription:
[0062]
[0063] Its preparation method:
[0064] a. Accurately weigh 3.3g of ciprofloxacin hydrochloride, 0.33g of sodium acetate, 0.42g of acetic acid, and 22.0g of glycerin, add 200mL of water for injection to dissolve, stir evenly, and dilute to 1000mL with water for injection to obtain a medicinal solution.
[0065] b. Filter the medicinal solution prepared above through a 0.45 μm microporous membrane once, and then through a 0.22 μm microporous membrane once.
[0066] c. The filtered medicinal solution is sterilized at 121° C. for 8 minutes by autoclaving, and then cooled.
[0067] d. After the medicinal liquid is tested and passed, fill 0.8mL of the above medicinal liquid into a 1mL single-dose packaging plastic container in a class 100 environment, and seal it to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com