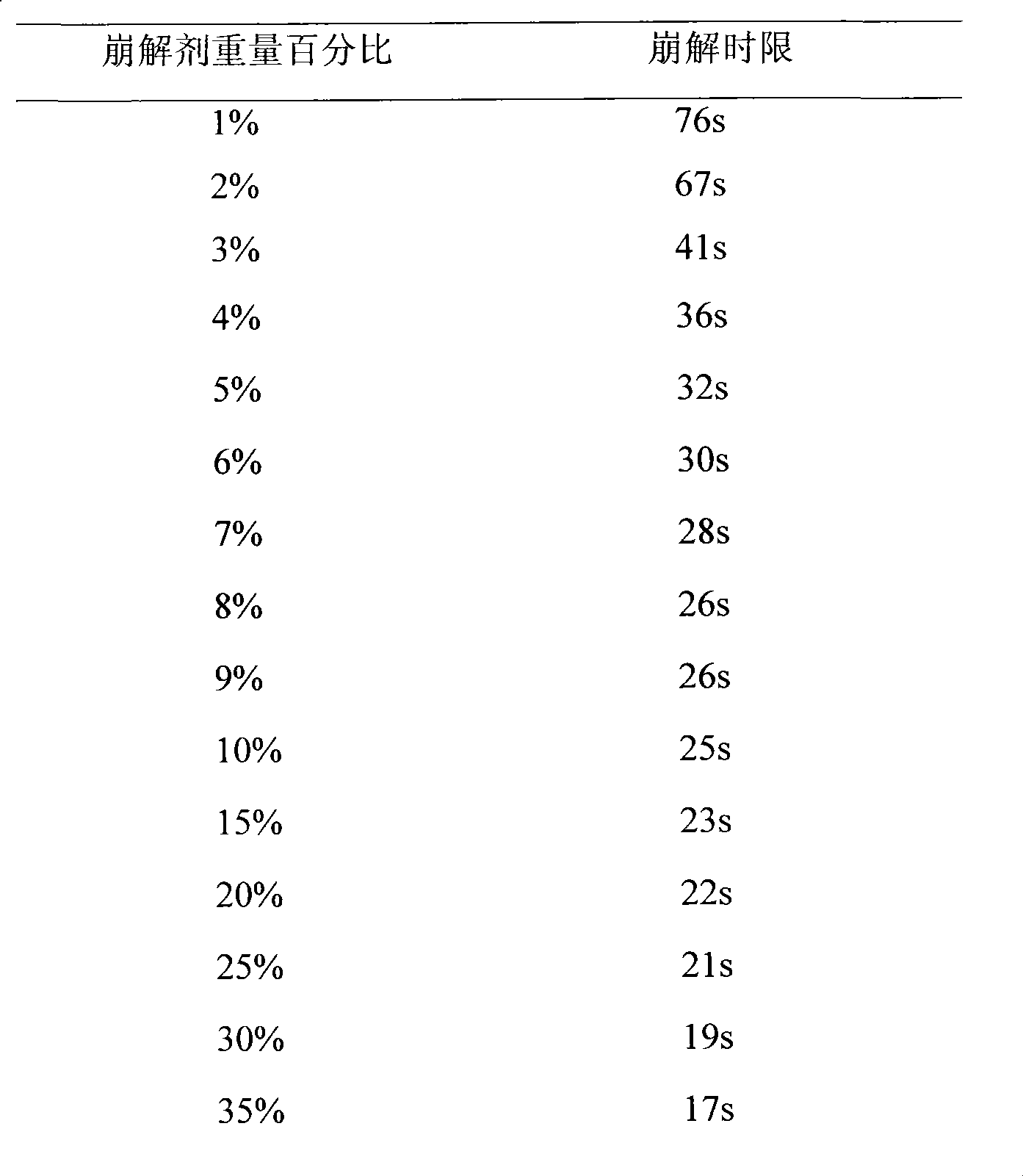Loratadine orally disintegrating tablet and technique for preparing the same
A technology of orally disintegrating tablets and loratadine, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pill delivery, which can solve problems such as inconvenient carrying, inconvenient, and affecting drug compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
Embodiment 2
[0052]
[0053] Preparation Process
[0054] (1) loratadine is crushed and passed through a 100-mesh sieve;
[0055] (2) Xylitol, starch and croscarmellose sodium are respectively sieved with 80 meshes:
[0056] (3) orange essence, ethyl cellulose, sucrose, magnesium stearate pass through 60 mesh sieves, respectively, for subsequent use;
[0057] (4) Place the loratadine, ethyl cellulose, starch, and xylitol of the recipe quantity in a rapid stirring granulator, and mix them uniformly according to the principle of equal increments;
[0058] (5) turn on stirring, add water to granulate;
[0059] (6) Ventilation drying at 40℃-60℃, 30 mesh granulation;
[0060] (7) Mix the sucrose, orange essence, and croscarmellose sodium in the prescribed proportions with the granules with a high-efficiency mixer;
[0061] (8) take by weighing the magnesium stearate of recipe quantity, mix with above-mentioned granule with high-efficiency mixer;
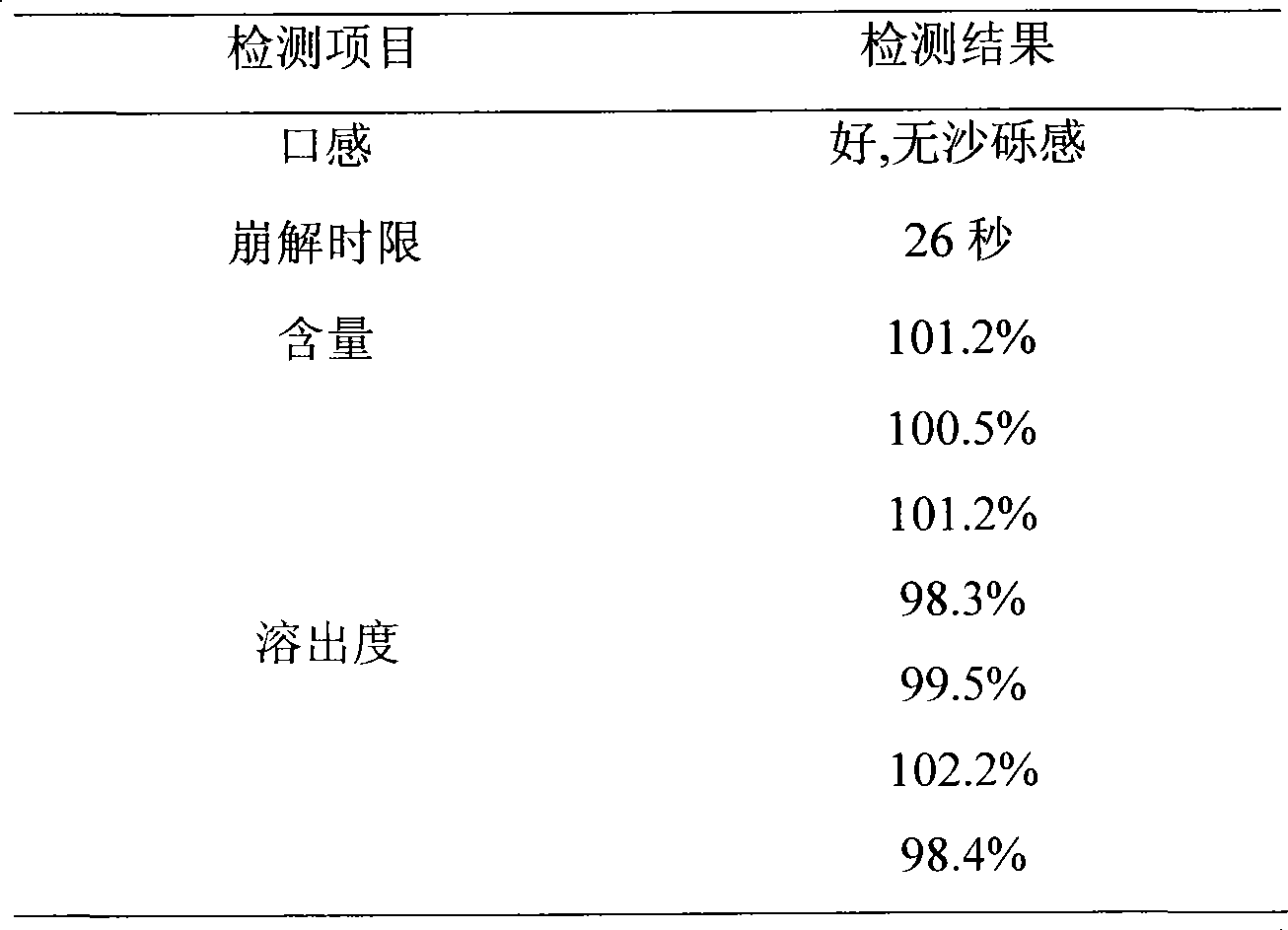
[0062] (9) Measure the content of the mai...
Embodiment 3
[0069]
[0070]
[0071] Preparation Process
[0072] (1) loratadine is crushed and passed through a 100-mesh sieve;
[0073] (2) Sorbitol, starch, crospovidone pass through 80 mesh sieves respectively:
[0074] (3) Grape essence, ethyl cellulose, stevioside, and micropowder silica gel are respectively sieved with a 60-mesh sieve, for subsequent use;
[0075] (4) Place the loratadine, ethyl cellulose, starch, and sorbitol of the recipe quantity in a rapid stirring granulator, and mix them uniformly according to the principle of equal increments;
[0076] (5) turn on stirring, add water to granulate;
[0077] (6) Ventilation drying at 40℃-60℃, 30 mesh granulation;
[0078] (7) Mix the stevioside, grape essence and crospovidone of the prescription ratio with the granules with a high-efficiency mixer;
[0079] (8) take by weighing the micropowder silica gel of recipe quantity, mix with above-mentioned particle with high-efficiency mixer;
[0080] (9) Measure the conten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com