Medicament composition containing losartan and hydrochlorothiazidum
A technology of hydrochlorothiazide and losartan potassium, which is applied in the direction of drug combination, medical preparations containing active ingredients, medical preparations without active ingredients, etc., can solve the problems of content reduction, increase of related substances, poor stability of hydrochlorothiazide, etc., and achieve The effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
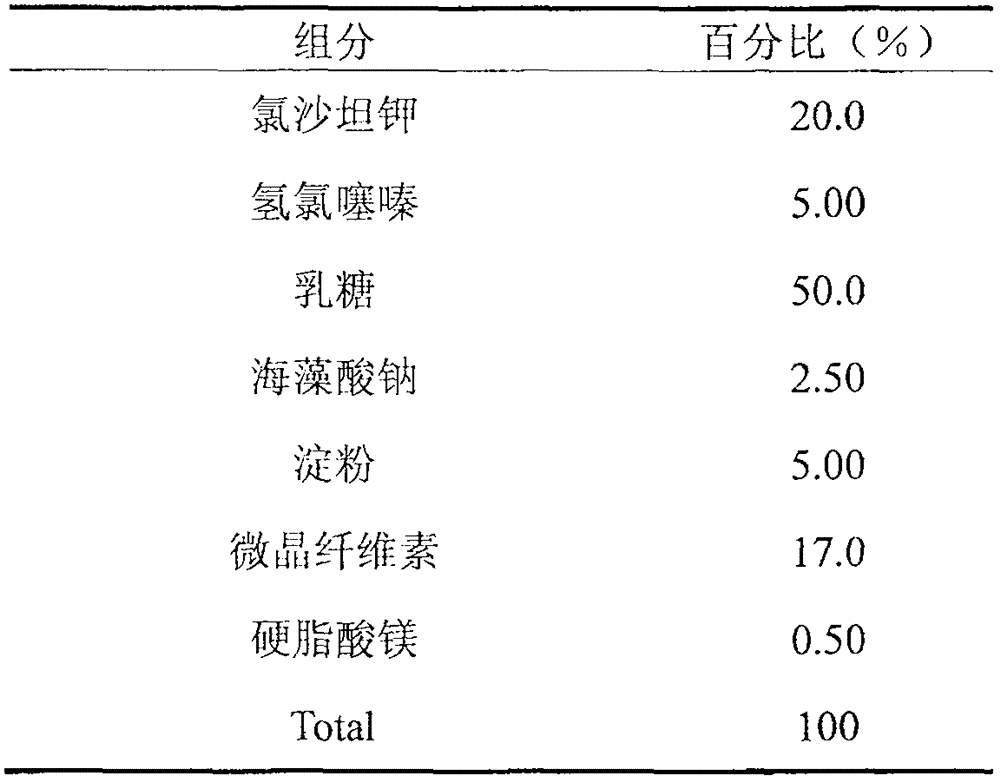
[0017]
[0018]
[0019] Preparation:
[0020] (1) Place the prescription amount of hydrochlorothiazide and lactose in a high-speed pulverizer to pulverize to obtain mixture I;
[0021] (2) Add prescription amounts of losartan potassium and sodium alginate to the mixture I in equal increments, and mix to obtain a pharmaceutical composition.
[0022] (3) adding prescription amount of starch and microcrystalline cellulose to the pharmaceutical composition to obtain mixture II;
[0023] (4) Add appropriate amount of wetting agent water to the mixture II, make soft material, pass through a 24-mesh sieve to granulate;
[0024] (5) After drying at 50°C, pass through a 30-mesh sieve for granulation;
[0025] (6) take magnesium stearate and dry granule and mix by recipe quantity to obtain mixture III;
[0026] (7) Fill the mixture III into the No. 1 capsule, and that's it.
Embodiment 2
[0028]
[0029] Preparation:
[0030] (1) Place the prescription amount of hydrochlorothiazide and lactose in a high-speed pulverizer to pulverize to obtain mixture I;
[0031] (2) Add prescription amounts of losartan potassium and sodium alginate to the mixture I in equal increments, and mix to obtain a pharmaceutical composition.
[0032] (3) adding prescription amount of starch and microcrystalline cellulose to the pharmaceutical composition to obtain mixture II;
[0033] (4) Add appropriate amount of wetting agent water to the mixture II, make soft material, pass through a 24-mesh sieve to granulate;
[0034] (5) After drying at 50°C, pass through a 30-mesh sieve for granulation;
[0035] (6) take magnesium stearate and dry granule and mix by recipe quantity to obtain mixture III;
[0036] (7) Compress mixture III on a tablet machine.
Embodiment 3
[0038]
[0039] Preparation:
[0040] (1) Place the prescription amount of hydrochlorothiazide and lactose in a high-speed pulverizer to pulverize to obtain mixture I;
[0041] (2) Add prescription amounts of losartan potassium and sodium alginate to the mixture I in equal increments, and mix to obtain a pharmaceutical composition.
[0042] (3) adding prescription amount sucrose and microcrystalline cellulose to the pharmaceutical composition to obtain mixture II;
[0043] (4) Add an appropriate amount of wetting agent water to the mixture II, make soft material, pass through a 16-mesh sieve and granulate;
[0044] (5) After drying at 50°C, sieve through a 30-mesh sieve for granulation, and pack them separately.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com