Novel method for synthesizing antineoplastic medicament carboplatin
An anti-tumor drug, carboplatin technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of difficult purification, long reaction process, poor product purity, etc., to achieve good product purity, easy operation, and process short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
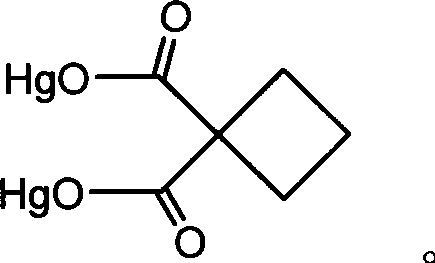
[0033] Example 1: 3 grams of 1,1-cyclobutanedicarboxylic acid and 1.6 grams of NaOH were added with an appropriate amount of water and stirred evenly, then mixed, and the pH value was adjusted to neutral, 11.6 grams of Hg 2 (NO 3 ) 2 2H 2 O is made into a solution, and the two solutions are mixed under stirring to obtain a white product precipitate, which is filtered, washed with water and absolute ethanol, and dried to obtain Hg 2 (1,1-CBDCA)(1,1-cyclobutanedicarboxymercurous acid) 6.71 g, yield 59.3%.
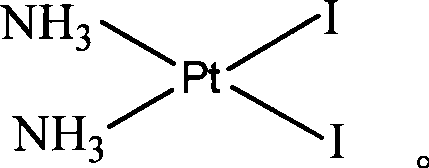
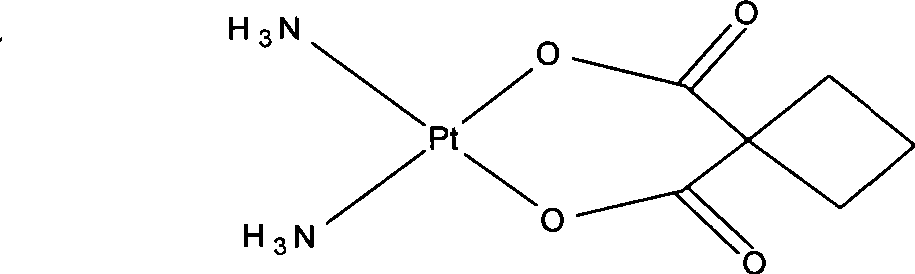
[0034] 5 g Pt(NH 3 ) 2 I 2 Add an appropriate amount of water and stir evenly with 180ml of purified water, add 5.6 g of mercurous 1,1-cyclobutanedicarboxylate, react in the dark at 55°C for 8 hours, filter to remove HgI precipitate, and concentrate the resulting solution under reduced pressure to obtain a white solid. Wash with water and absolute ethanol respectively, and dry to obtain 3.31 g of carboplatin solid product with a yield of 86.15%. The structure is consis...
Embodiment 2
[0040] Example 2: 3 grams of 1,1-cyclobutanedicarboxylic acid and 1.6 grams of NaOH were added with an appropriate amount of water and stirred evenly, then mixed, and the pH value was adjusted to neutral, 11.6 grams of Hg 2 (NO 3 ) 2 2H 2 O is made into a solution, and the two solutions are mixed under stirring to obtain a white product precipitate, which is filtered, washed with water and absolute ethanol, and dried to obtain Hg 2 (1,1-CBDCA) 7.19 g, yield 61.5%.
[0041] 5 g Pt(NH 3 ) 2 I 2Add 200ml of purified water and stir evenly, add 5.6g of mercurous 1,1-cyclobutanedicarboxylate, react in the dark at 60°C for 10 hours, filter to remove HgI precipitate, concentrate the obtained solution under reduced pressure to obtain a white solid, filter The precipitated white solid was washed with water and absolute ethanol respectively, and dried to obtain 3.34 g of carboplatin as a solid product with a yield of 86.79%. The structure is consistent with the target compound by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com