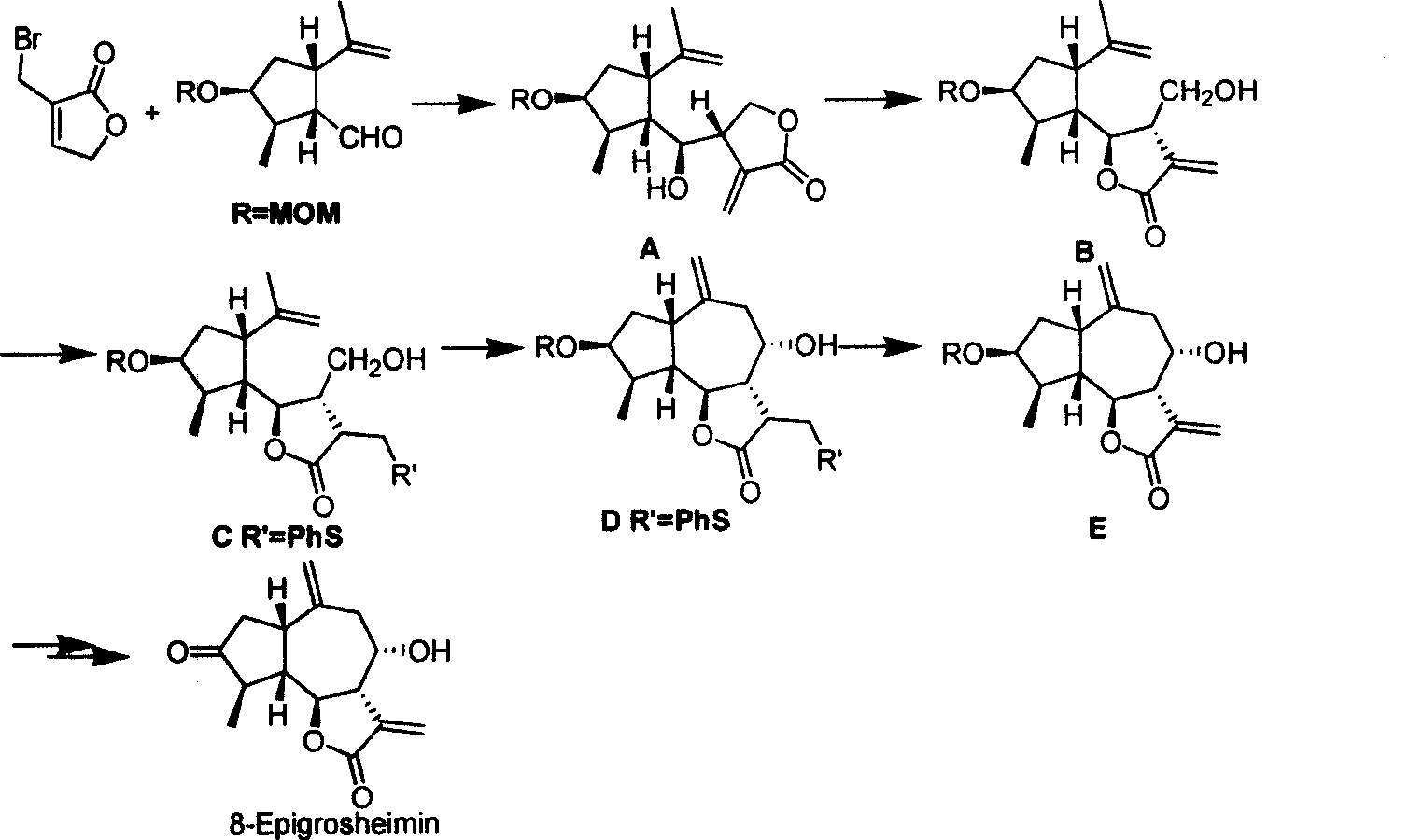
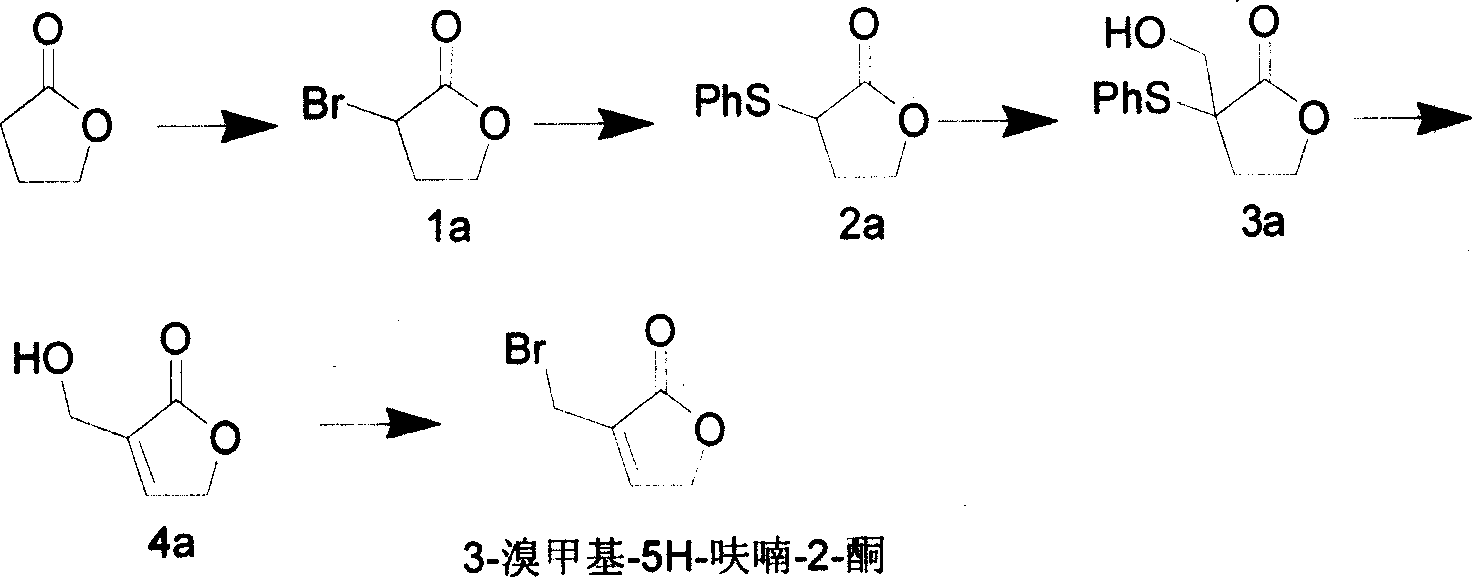
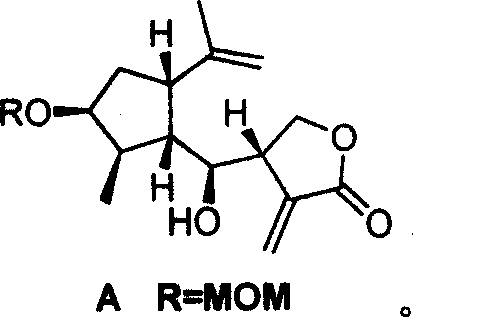
Method for synthesizing natural sesquiterpene Crepis virens extract (8-Epigrosheimin)
A sesquiterpene lactone and synthetic method technology, applied in the field of chemical synthesis, can solve the problems of large side effects, carcinogenicity, poor effect, etc., and achieve the effect of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0028] The synthesis of key intermediate 2-methyl-3-methoxymethyl-5-isopropenyl-cyclopentanecarbaldehyde (5-Isopropenyl-3-methoxymethoxy-2-methyl-cyclopentanecarbaldehyde) of the present invention refers to literature method to synthesize, the yield is 50%. (Lee E, Yoon C H. Stereoselective favorskii rearrangement of carvone chlorohydrin: Expedient synthesis of (+)-dihydrone petalactone and (+)-iridomyrmecin. JChem S, Chem Commun, 1994: 479~481)
[0029] 1. Synthesis of intermediate 3-bromomethyl-5H-furan-2-one:
[0030]
[0031] Synthesis of Compound 1a
[0032]Add 42g of 1,4-butyrolactone and 5.7g of red phosphorus to a 500mL four-neck flask (thermometer, reflux condenser, dropping funnel, electromagnetic stirrer), stir for 0.5h, then add 28mL of liquid bromine under an ice bath, and dropwise Afterwards, the temperature rose to 70°C, and 28 mL of Br2 was added dropwise again. After the drop, the temperature rose to 80°C, reacted for 3 hours, cooled, blown in air, and to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com