Pyrethroid compounds, preparation and use thereof
A compound and mixture technology, applied in the field of sanitary pest control, can solve the problems of increasing production cost, increasing environmental burden, increasing the amount of pyrethroid raw materials, etc., and achieves the effect of quickly killing pests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
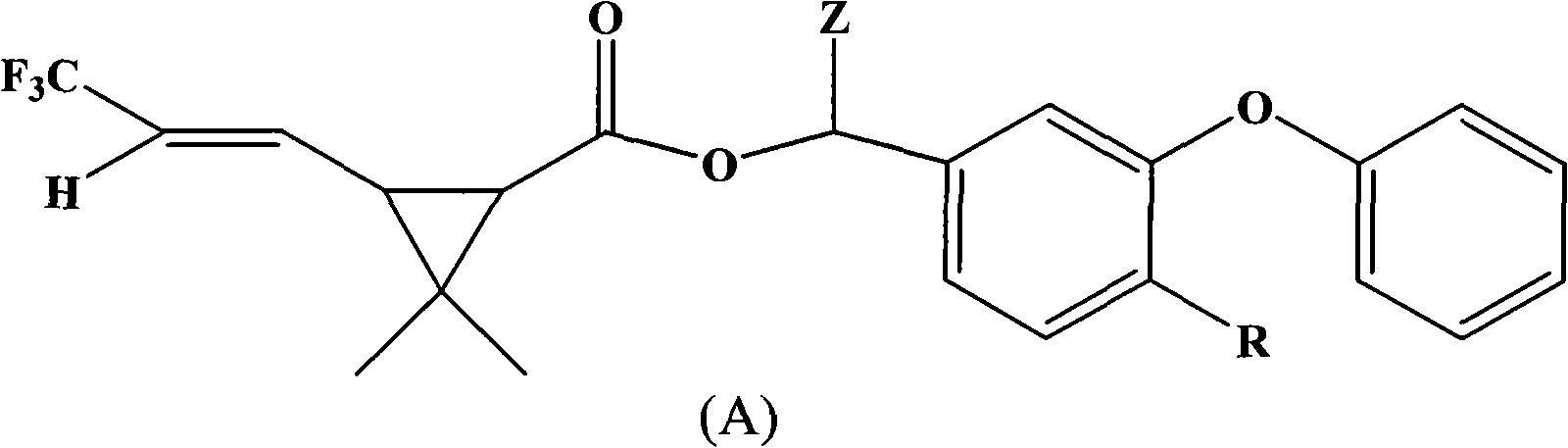
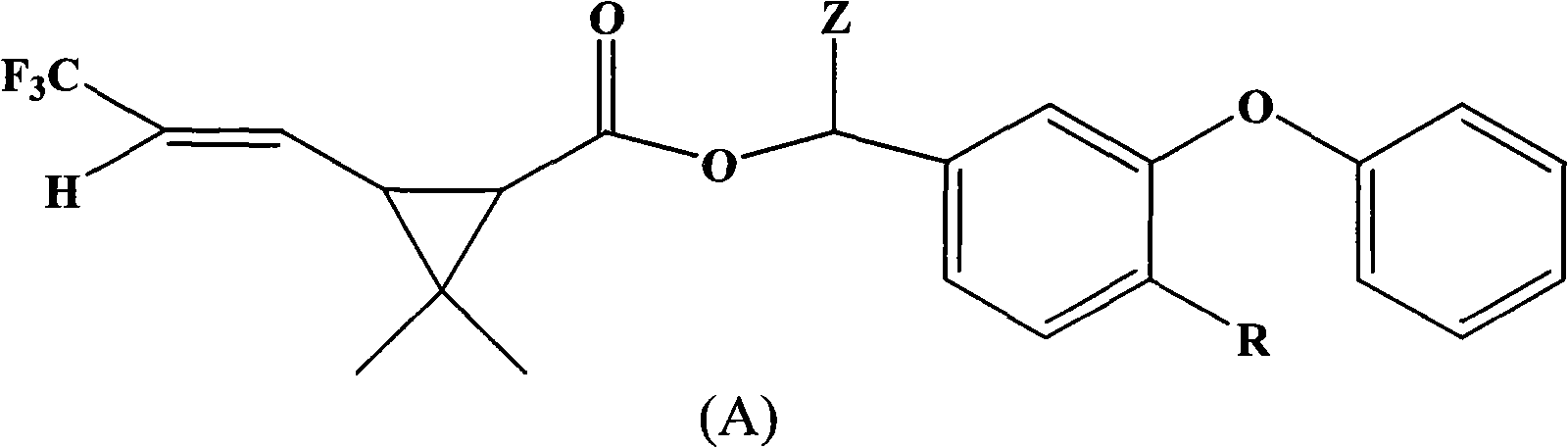
[0047] Preparation Example 1: Compound (1) α-cyano-3-phenoxy-4-fluoro-benzyl-2,2-dimethyl-3-(2-trifluoromethylvinyl)cyclopropanecarboxy Synthesis of esters
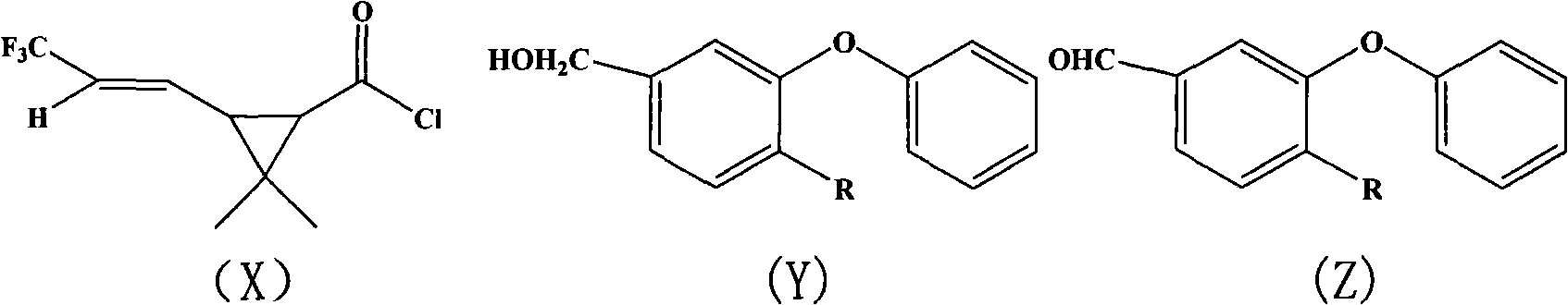
[0048] In a 200ml four-necked bottle, put 10.8g (0.05mol) of 4-fluoro-3-phenoxybenzaldehyde, 5g of pyridine, dissolve in 80ml of cyclohexane, dropwise add 2.45g (0.05mol) of NaCN Saturated aqueous solution, stirring for 10 minutes after throwing in, slowly adding 2,2-dimethyl-3-(2-trifluoromethylvinyl)cyclopropanecarboxylic acid chloride 13.6g (0.06mol ), the dropwise temperature rises to 30°C for 4 hours of reaction. The organic phase was successively washed once with 50ml 5% sodium hydroxide solution, 5% hydrochloric acid and saturated brine, and the oil layer was separated and heated to 100°C under a negative pressure of 10mmHg to remove the solvent to obtain the compound α-cyano-3-phenoxy Dimethyl-4-fluoro-benzyl-2,2-dimethyl-3-(2-trifluoromethylvinyl)cyclopropanecarboxylate 20.2, content 94.3%, yield 88.1% (calcula...
preparation Embodiment 2
[0049] Preparation Example 2: Synthesis of Compound (2) α-cyano-3-phenoxybenzyl-2,2-dimethyl-3-(2-trifluoromethylvinyl)cyclopropanecarboxylate
[0050] In a 200ml four-necked bottle, drop 9.9g (0.05mol) of 3-phenoxybenzaldehyde, 5g of pyridine, be dissolved in 80ml cyclohexane, add dropwise the saturated aqueous solution containing 2.45g (0.05mol) NaCN, and then Add 0.5g of tetrabutylammonium bromide, stir for 10min after throwing in, slowly add 2,2-dimethyl-3-(2-trifluoromethylvinyl)cyclopropanecarboxylate dropwise within 2 hours at 10-20°C 13.6 g (0.06 mol) of acid chloride was added dropwise to 30°C for 4 hours. The organic phase was successively washed once with 50ml 5% sodium hydroxide solution, 5% hydrochloric acid and saturated brine, and the oil layer was separated and heated to 100°C under a negative pressure of 10mmHg to remove the solvent to obtain the compound α-cyano-3-phenoxy 19.85% benzyl-2,2-dimethyl-3-(2-trifluoromethylvinyl)cyclopropanecarboxylate, content 9...
preparation Embodiment 3
[0051] Preparation Example 3: Synthesis of Compound (3) 3-phenoxy-4-fluoro-benzyl-2,2-dimethyl-3-(2-trifluoromethylvinyl)cyclopropanecarboxylate
[0052] In a 200ml four-necked bottle, put 10.9g (0.05mol) of 4-fluoro-3-phenoxybenzyl alcohol, 5g of pyridine, dissolve in 80ml of toluene, stir after throwing in, and slow down within 2 hours at 10-20°C. 13.6 g (0.06 mol) of 2,2-dimethyl-3-(2-trifluoromethylvinyl)cyclopropanecarboxylic acid chloride was slowly added dropwise, and the temperature was raised to 30° C. for 4 hours after the dropwise reaction. The organic phase was successively washed once with 50ml of 5% sodium hydroxide solution, 5% hydrochloric acid and saturated brine, and the separated oil layer was heated to 100°C under a negative pressure of 10mmHg to remove the solvent toluene to obtain the compound 3-phenoxy-4- Fluoro-benzyl-2,2-dimethyl-3-(2-trifluoromethylvinyl)cyclopropane carboxylate 18.82, content 96.9%, yield 89.9% (calculated as aldehyde). The molecula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com