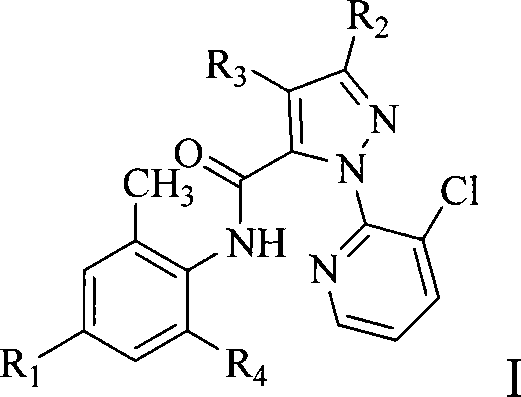
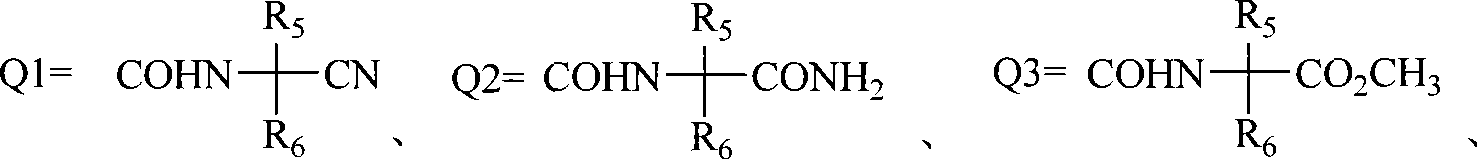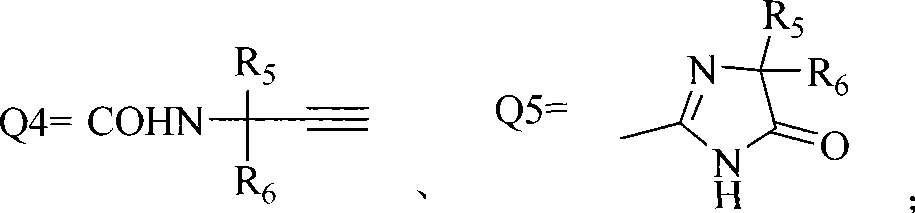Anthranilic acid compound and use thereof
A technology of anthranilic acid and compounds, applied in the field of anthranilic acid compounds, as pesticides, salts and compositions, and N-oxides, can solve the problems of reduced productivity and increased consumer costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0122] Example 1: Preparation of Compound 1-152
[0123]
[0124] Take 0.5 g of II-I in a 50 ml reaction flask, add 25 ml of tetrahydrofuran, and add 1 g of HOCH under stirring 2 CH 2 N(CH 3 ) 2 , heated to reflux for 1 hour. After TLC monitors the completion of the reaction, after desolvation under reduced pressure, pour 50 milliliters of saturated saline into the reaction flask, extract three times with 60 milliliters of ethyl acetate, dry, and desolventize, and column chromatography gives 0.31 grams of the product, namely the compound 1-152, yield 51.8%. The melting point is 80-82°C.
[0125] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0126] Compound 1-152: δppm 2.18 (3H, s), 2.41 (6H, s), 2.79 (2H, t), 4.45 (2H, t), 7.19 (1H, s), 7.39 (2H, m), 7.80- 7.88 (2H, m), 8.45 (1H, q), 10.14 (1H, s).
example 2
[0127] Example 2: Preparation of compound 2-2
[0128]
[0129] Take 1 g of IV-1 in a 100 ml reaction bottle, add 50 ml of dioxane and intermediate H 2 NC(CH 3 ) 2 CN, reflux reaction for 3 hours. After the completion of the reaction as monitored by TLC, after desolvation under reduced pressure, 50 ml of saturated saline was poured into the reaction flask, extracted three times with 90 ml of ethyl acetate, dried, and desolvated, and column chromatography gave III-1 intermediate 0.63 gram.
[0130] The prepared intermediate III-1 was dissolved in 60 ml of tetrahydrofuran, 1 ml of triethylamine was added, 1.02 g of acid chloride was added dropwise under stirring, and the reaction was carried out at room temperature for 4 hours. After the reaction was monitored by TLC, 0.45 g of the product was obtained by column chromatography , namely compound 2-2, the yield is 31%. The melting point is 170-172°C.
[0131] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl...
example 3
[0133] Example 3: Preparation of Compound 2-6
[0134]
[0135] Take 0.5 g of II-2 in a 50 ml reaction flask, add 25 ml of acetonitrile, and add 0.8 g of H 2 NC(CH 3 ) 2 CN, heated up and refluxed for 3 hours. After TLC monitors the completion of the reaction, after desolvation under reduced pressure, pour 50 milliliters of saturated saline into the reaction flask, extract three times with 90 milliliters of ethyl acetate, dry, and desolventize, and obtain 0.27 grams of product by column chromatography, namely compound 2-6, yield 45.3%. The melting point is 140-142°C.
[0136] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0137] Compound 2-6: δppm 1.95 (6H, s), 2.65 (3H, s), 6.85 (1H, s), 7.28 (1H, q), 7.87 (1H, s), 7.92 (1H, d), 8.30 ( 1H,q), 8.48(1H,s).
[0138] Other compounds of general formula I can be prepared by the preparation methods provided by the present invention.
[0139] Melting point and nuclear magnetic data ( 1 H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com