Malt oligosaccharide based mycose synthetase, coding gene and use
A technology of trehalose synthase and maltooligosaccharide base, applied in the field of maltooligosaccharide base trehalose synthase and its coding gene and application, can solve the problem of high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
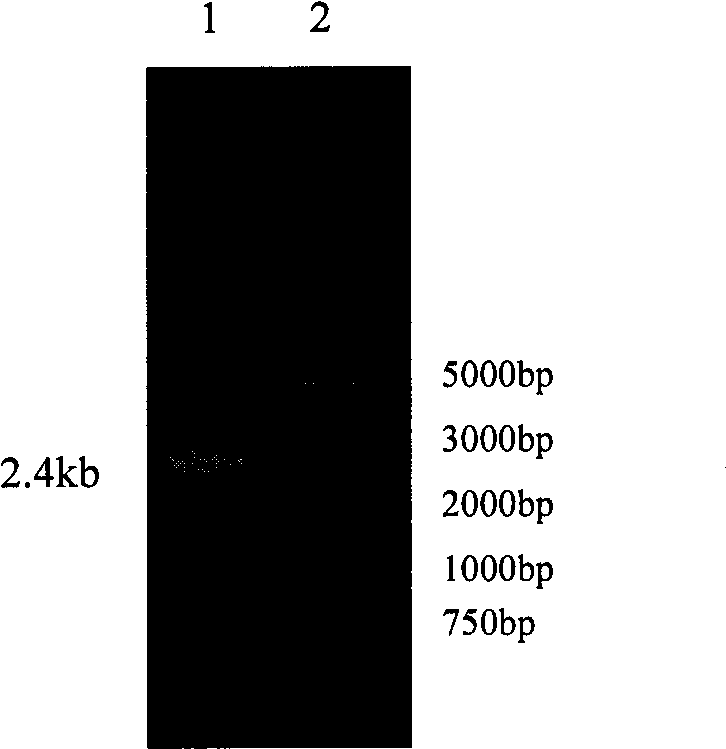
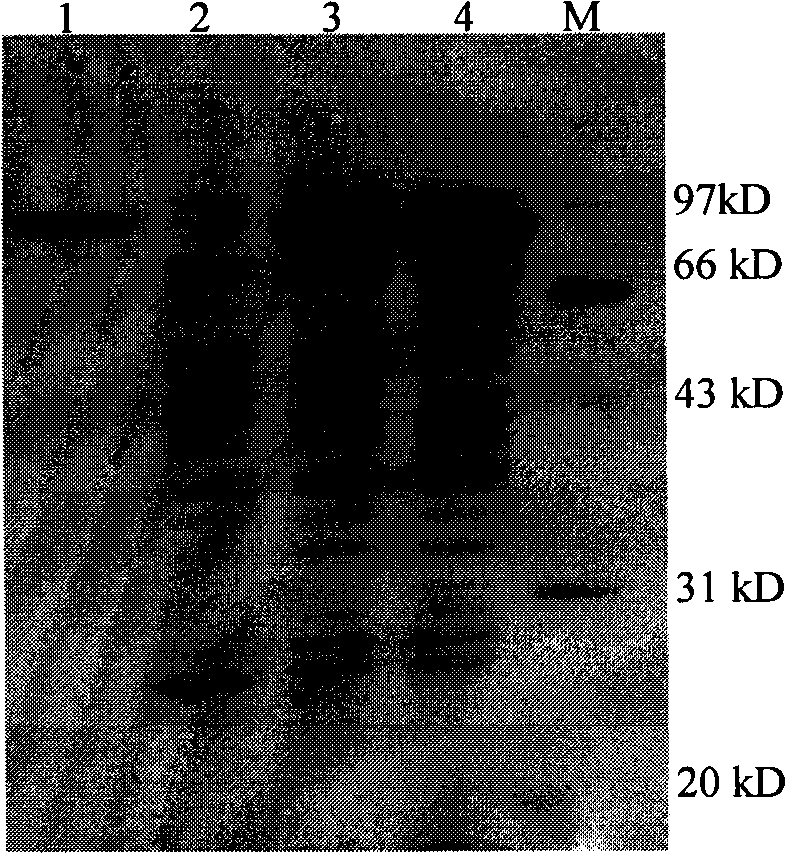
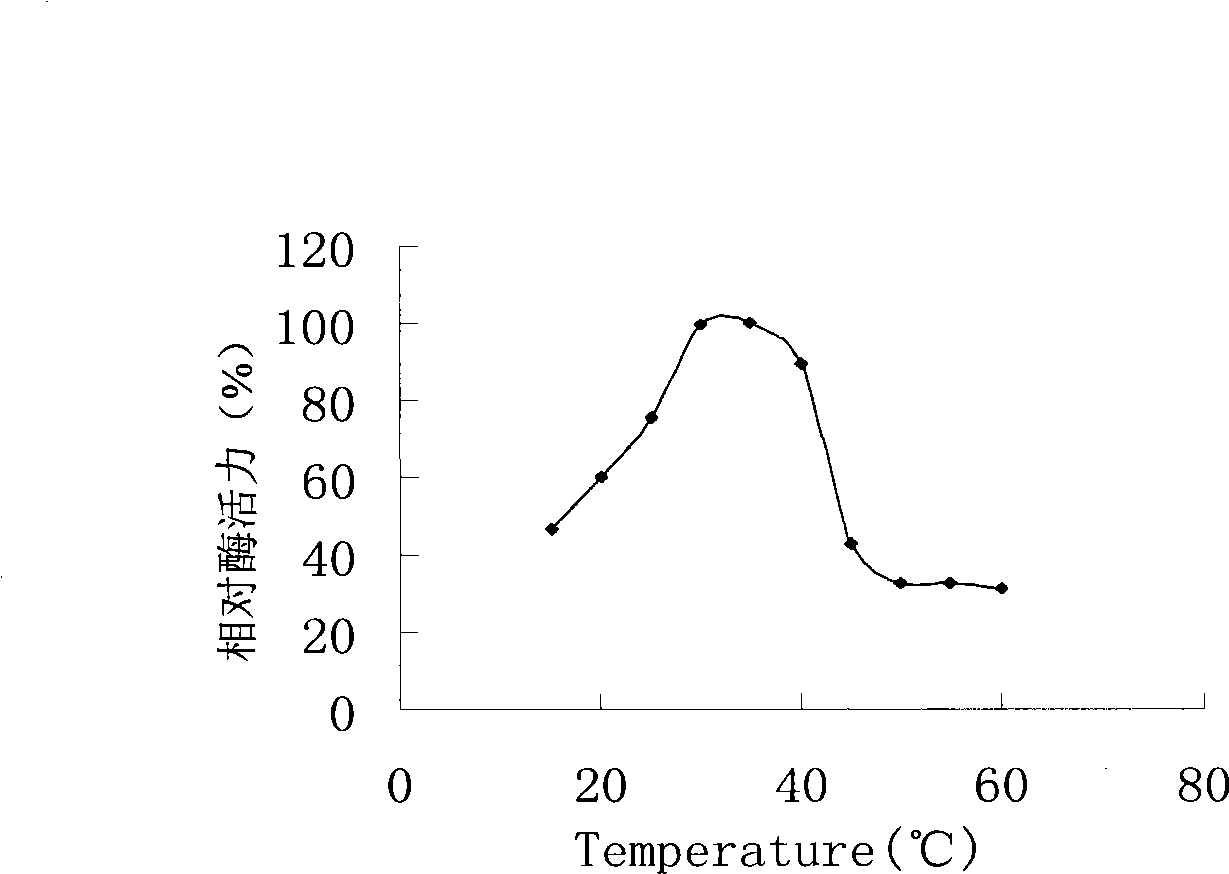
[0048] Embodiment 1, maltooligosaccharide-based trehalose synthase
[0049] 1. Acquisition of maltooligosaccharyl trehalose synthase (MTSase-1) and its coding gene (TreY-1)
[0050] Design PCR amplification primers, upstream primer TreY-1F: 5′ CCATGG ATGGCACGTCCAATTTCC 3′, downstream primer TreY-1R: 5′ CCATGG AAACTCACTATCGGGTACTAAAAC3' wherein NcoI restriction sites were introduced into the upstream and downstream primers respectively, as shown in the underline, so that the target fragment could be inserted into the expression vector pET30a treated with the same restriction enzyme digestion after NcoI restriction.
[0051] Using TreY-1F and TreY-1R as primers, and using Corynebacterium glutamicum (Corynebacterium glutamicum) CGMCC1.1886 genomic DNA as a template, a PCR reaction was performed. The reaction conditions were as follows: 5 minutes of pre-denaturation at 94°C, denaturation at 94°C for 20s, annealing at 56°C for 20s, extension at 72°C for 3min and 20s, and 30 cyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com