Qualitative and quantitative analysis method for hydrogenated tail-oil long and straight side chain cycloparaffin and alkylbenzene
A technology for the quantitative analysis of paraffins, applied in analytical materials, measuring devices, material separation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
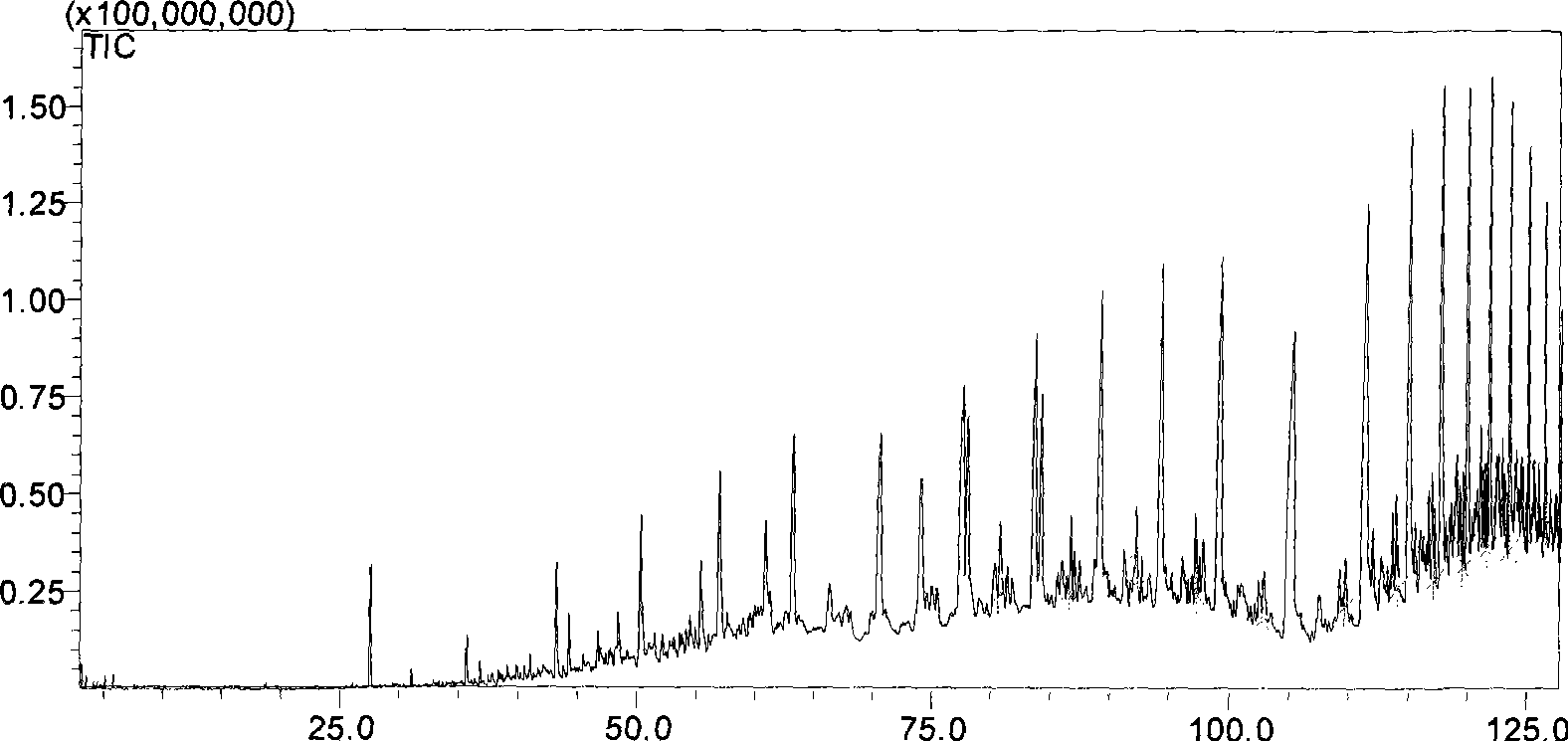
[0073] Sample: HVGO1 was diluted 10 times with n-hexane, and Shimadzu's GCMS-QP2010 (EI) instrument was used. The temperature of the injection port is 250°C, helium is used as the carrier gas, the carrier gas flow rate is 1ml / min, the sample is injected in a split mode, the split ratio is 15:1, the chromatographic column: HP-5ms 60m×0.32mm×0.25um, the programmed temperature control is Keep at 25°C for 8 minutes, raise to 60°C at 4°C / min and keep for 10 minutes, rise to 200°C at 1°C / min and keep for 15 minutes, rise to 323°C at 4°C / min and keep for 35 minutes. The ion source temperature is 200°C, the interface temperature is 280°C, the injection volume is 1ul, and the spectra are collected in SIM mode.
Embodiment 2
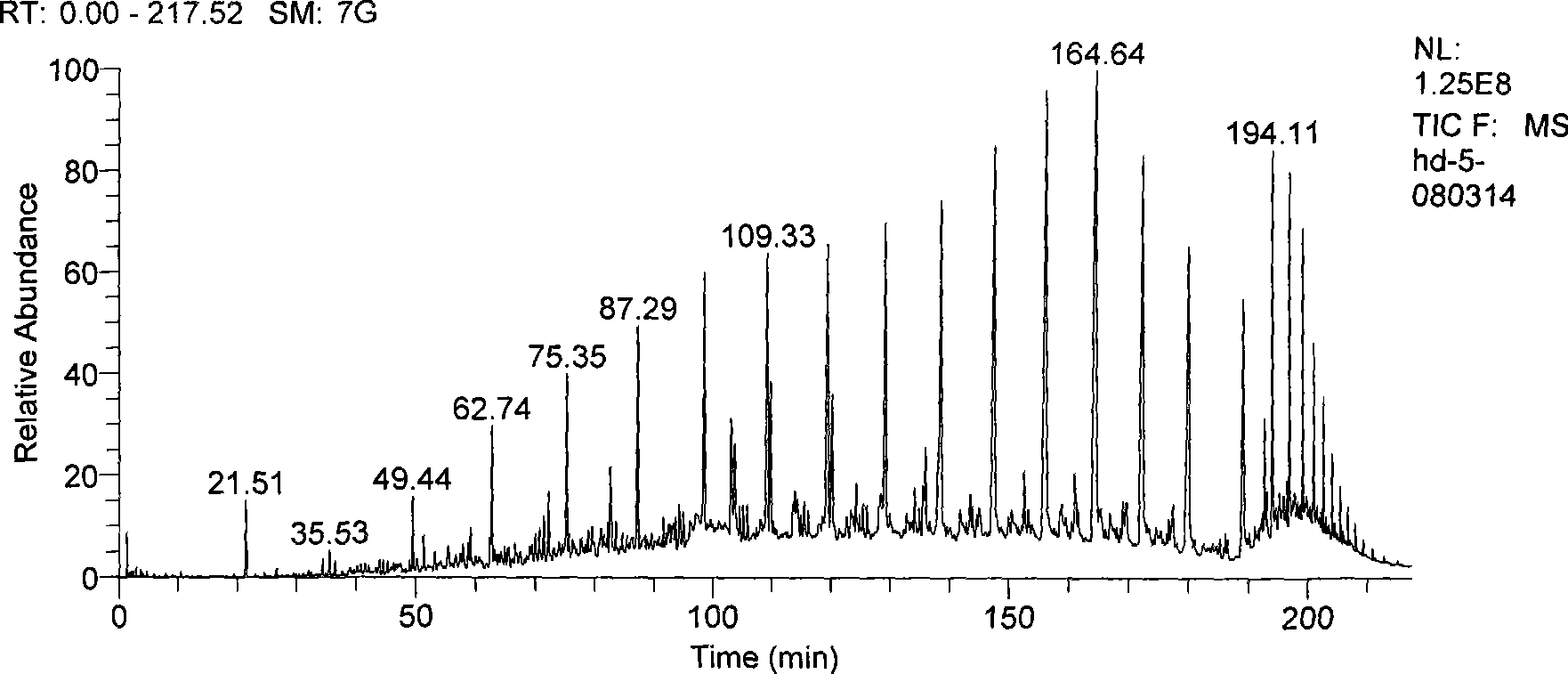
[0075] Sample: HVGO2 was diluted 10 times with n-hexane, using Shimadzu's GCMS-QP2010(EI) instrument. The temperature of the injection port is 260°C, helium is used as the carrier gas, the carrier gas flow rate is 1.5ml / min, the sample is injected in a split flow mode, the split ratio is 18:1, the chromatographic column: HP-5ms 60m×0.32mm×0.25um, the temperature is controlled by the program Keep at 35°C for 10 minutes, rise to 40°C at 2°C / min and hold for 4 minutes, rise to 200°C at 1°C / min and hold for 8 minutes, rise to 300°C at 5°C / min and hold for 33 minutes. The ion source temperature is 200°C, the interface temperature is 300°C, the injection volume is 1ul, and the spectra are collected in SIM mode.
Embodiment 3
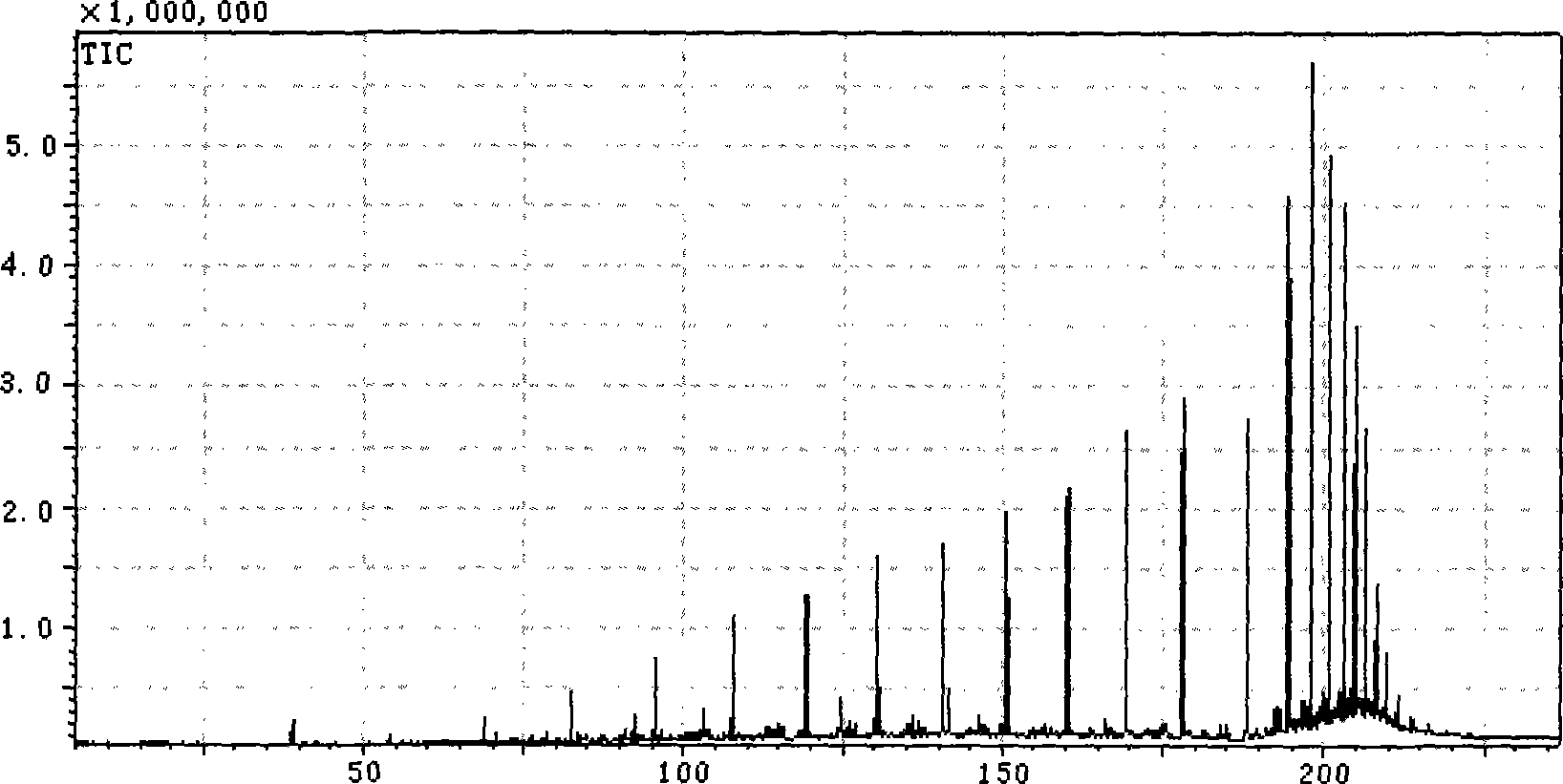
[0077] Sample: HVGO3 was diluted 10 times with n-hexane, using Shimadzu's GCMS-QP2010(EI) instrument. The temperature of the injection port is 270°C, helium is used as the carrier gas, the carrier gas flow rate is 2ml / min, the sample is injected in a split flow mode, the split ratio is 20:1, the chromatographic column: HP-5ms 60m×0.32mm×0.25um, the programmed temperature control is Keep at 35°C for 9 minutes, rise to 40°C at 2°C / min and hold for 5 minutes, rise to 200°C at 1°C / min and hold for 9 minutes, rise to 300°C at 5°C / min and hold for 32 minutes. The ion source temperature is 200°C, the interface temperature is 300°C, the injection volume is 1ul, and the spectra are collected in SIM mode.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com