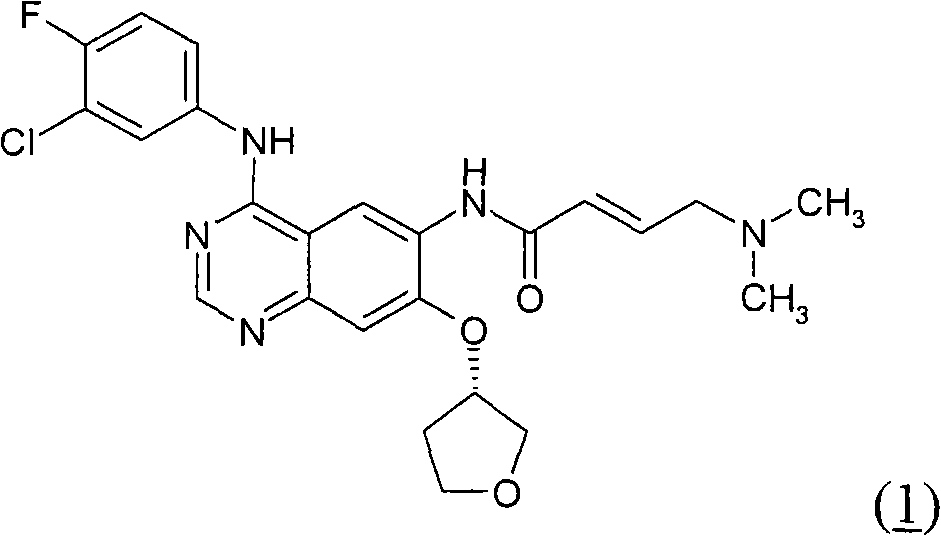
Method for treating cancer harboring EGFR mutations
A cancer and radiation therapy technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., and can solve the problem that the precise mechanism is not fully understood.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0173] Example 1: Coated tablet containing 75 mg of active substance
[0174] 1 tablet contains:
active substance
75.0mg
93.0mg
35.5mg
10.0mg
Hydroxypropylmethylcellulose
15.0mg
1.5mg
230.0mg
[0175] preparation:
[0176] The active substance is mixed with calcium phosphate, corn starch, polyvinylpyrrolidone, hydroxypropylmethylcellulose and half the prescribed amount of magnesium stearate. Billets with a diameter of 13 mm are prepared on a tablet machine and, using suitable equipment, these billets are rubbed through a sieve with a mesh size of 1.5 mm and mixed with the rest of the magnesium stearate. The granulation is compressed in a tablet machine to form tablets of the desired shape.
[0177] Weight of tablet core: 230mg
[0178] Mold: 9mm, convex
[0179] The tablet cores thus obtained are coated with ...
Embodiment 2
[0181] Example 2: Tablets containing 100 mg of active substance
[0182] 1 tablet contains:
active substance
100.0mg
80.0mg
34.0mg
4.0mg
Magnesium stearate
2.0mg
220.0 mg
[0183] preparation:
[0184] The active substance, lactose and starch are mixed together and moistened evenly with an aqueous solution of polyvinylpyrrolidone. After the wet composition was sieved (mesh size 2.0 mm) and dried in a rack-type dryer at 50° C., it was sieved again (mesh size 1.5 mm) and a lubricant was added. The final blend is compressed to form tablets. .
[0185] Tablet weight: 220mg
[0186] Diameter: 10mm, double-sided, faceted on both sides and notched on one side
Embodiment 3
[0187] Example 3: Tablets containing 150 mg of active substance
[0188] 1 tablet contains:
[0189] active substance
150.0mg
powdered lactose
89.0 mg
corn starch
40.0mg
10.0mg
10.0mg
Magnesium stearate
1.0mg
300.0mg
[0190] preparation:
[0191] The active substance mixed with lactose, corn starch and silicon dioxide is moistened with a 20% aqueous solution of polyvinylpyrrolidone and passed through a sieve with a mesh size of 1.5 mm. The granules dried at 45°C were again passed through the same sieve and mixed with the prescribed amount of magnesium stearate. The mixture is compressed into tablets.
[0192] Tablet weight: 300mg
[0193] Mold: 10mm, flat
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com