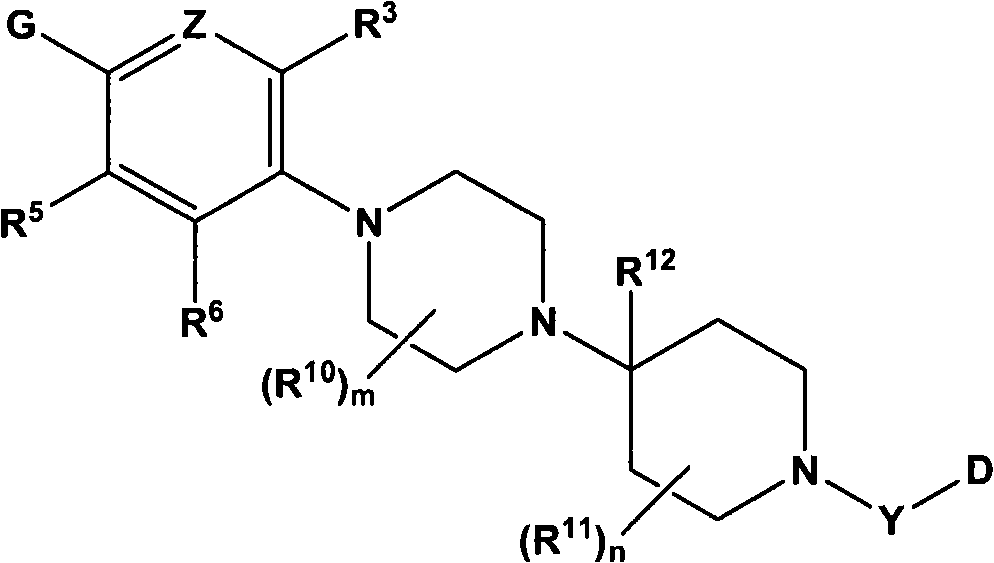
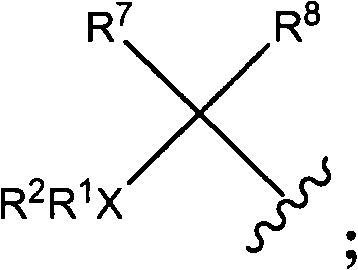
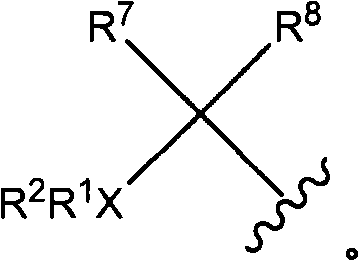Heterocyclic substituted piperazine compounds with CXCR3 antagonist activity
A compound and solvate technology, applied in the field of treating diseases and diseases involving CXCR3, can solve the problem of not showing such effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0401]
[0402] To a cold (-78°C) 1000ml round bottom flask containing 200ml methanol was added dropwise thionyl chloride (10ml, 140mmol) followed by 5-bromopyridinecarboxylic acid (15g, 75mmol). After stirring at -78°C for 10 minutes, the reaction was allowed to warm to room temperature and stirred for 16 hours. Excess thionyl chloride and methanol were removed in vacuo to give A2 (18 g, 95%) as a light brown solid. M+H=216
Embodiment 2
[0404]
[0405] A 250 ml round bottom flask was charged with A2 (4 g, 15.9 mmol), 1-Boc-2-S-ethylpiperazine A3 (prepared according to Kiley et al., Org. Prep. Proc. Int. 1990, 22, 761; 4.2 g, 18.6 mmol), tris(dibenzylideneacetone)dipalladium (340 mg, 0.37 mmol), rac-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (BINAP ) (495 mg, 0.74 mmol), cesium carbonate (12 g, 37.2 mmol) and toluene (80 ml). After heating the mixture at 100°C for 16 hours, fresh tris(dibenzylideneacetone)dipalladium (340 mg, 0.37 mmol) and BINAP (495 mg, 0.74 mmol) were added and heating continued for 3 days. The solvent was removed in vacuo and the residue was suspended in 100 ml of ethyl acetate. The mixture was extracted with water and brine, dried over sodium sulfate, and concentrated in vacuo. The residue was purified via silica gel flash chromatography (5% methanol / 95% DCM) to yield 5.3 g of partially purified material which was used directly in the next step. M+H=350
Embodiment 3
[0407]
[0408] Intermediate A4 (5.28 g, 15.1 mmol) was stirred with N-bromo-succinimide (5.4 g, 30.2 mmol) in 30 ml DMF at room temperature for 24 hours. The solvent was removed in vacuo and the residue was purified directly by flash chromatography on silica gel eluting with 50% ethyl acetate / 50% hexanes to afford A5 (1.22 g, 19% yield, M+H=428). The column was further eluted with 10% 7N ammonia in methanol / 90% DCM to afford A6 (1.28 g, 25%, M+H=328).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com