Antibody having enhanced adcc activity and method for production thereof
A technology of antibody and humanized antibody, applied in the direction of antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, hybrid cell preparation, etc., can solve the problem of ADCC activity increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
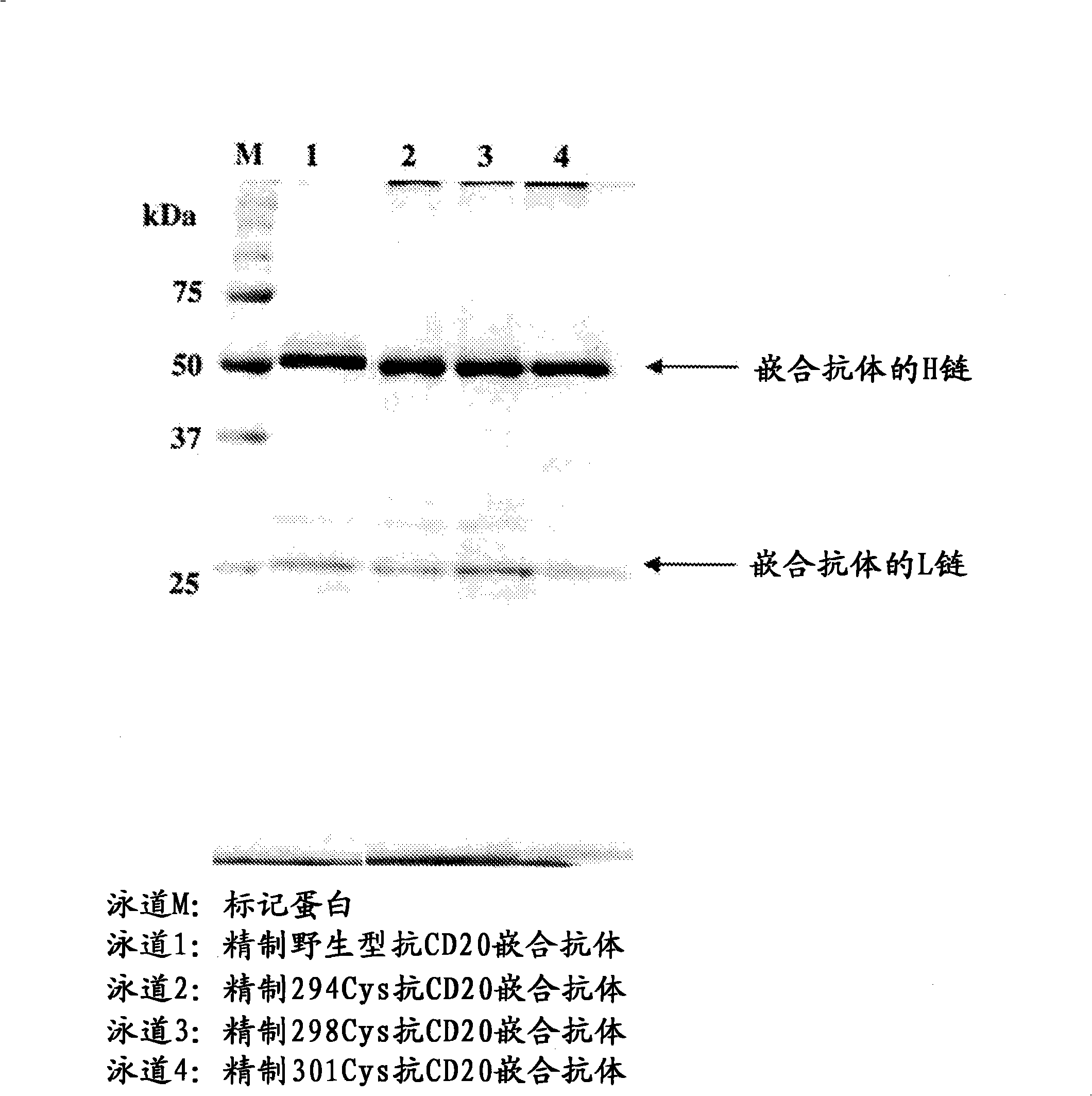
[1113]Anti-CD20 chimeric antibodies of 293, 294, 298, 299, 300, and 301 Cys types were prepared, and the ADCC activities of these various Cys type Anti-CD20 chimeric antibodies were evaluated. As a result, compared to the ADCC activity of the wild-type Anti-CD20 chimeric antibody, the 294, 298, and 301Cys-type Anti-CD20 chimeric antibodies showed very high ADCC activity.
[1114] The following describes the preparation methods of the wild-type and three variants (294Cys, 298Cys, and 301Cys) of the Anti-CD20 chimeric antibody, as well as its ADCC activity measurement and reactivity to CD20 molecules.
[1115] 1) Production of Anti-CD20 chimeric antibody
[1116] The chimeric antibody undergoes the following steps A) to F) to obtain a purified chimeric antibody.
[1117] A) Cloning of genes necessary for making chimeric antibodies;
[1118] B) The mutation introduction of the cloned gene;
[1119] C) Constructing a chimeric antibody expression vector combining the cloned gene and ...
Embodiment 2
[1392] Cys-type Anti-CD20 chimeric antibodies of 290, 291, 292, 302, and 303 were prepared by the same method as in Example 1, and the ADCC activities of these various Cys-type Anti-CD20 chimeric antibodies were evaluated. As a result, compared with the ADCC activity of the wild-type Anti-CD20 chimeric antibody, the 290, 291, 292, 302, and 303 Cys type Anti-CD20 chimeric antibodies showed very high ADCC activity.
[1393] The following describes the preparation methods of the wild-type and five variants (290Cys, 291Cys, 292Cys, 302Cys, and 303Cys) of the Anti-CD20 chimeric antibody, as well as its ADCC activity measurement and reactivity to CD20 molecules.
[1394] 1) Production of Anti-CD20 chimeric antibody
[1395] The chimeric antibody undergoes the following steps A) to F) to obtain a purified chimeric antibody.
[1396] A) Cloning of genes necessary for making chimeric antibodies;
[1397] B) The mutation introduction of the cloned gene;
[1398] C) Construction of a chime...
Embodiment 3
[1524] (Production of Anti-EGFR Humanized Antibody)
[1525] In order to express wild-type and 298Cys-type Anti-EGFR humanized antibodies in CHO cells, three kinds of expression vectors (Anti-EGFR humanized antibody L chain expression vector, wild-type Anti-EGFR humanized Antibody H chain expression vector, 298Cys type Anti-EGFR humanized antibody H chain expression vector). The details of the construction of each expression vector are described below.
[1526] 1) Construction of Anti-EGFR humanized antibody L chain expression vector
[1527] Using the gene containing the variable and constant regions of the human-type Anti-EGFR antibody L chain as a template, using the primers shown below, a DNA fragment was obtained by PCR.
[1528] EGFR-L1 primer: ACCGCTCGAGATGGACATGAGGGTCCCCGCTCAGCTC (serial number 220)
[1529] EGFR-L2 primer: ATAGTTTAGCGGCCGCTTACGAACATTCTGTAGGGGCCACTGTCTT (serial number 221)
[1530] The DNA fragment obtained in the PCR reaction was purified by ethanol preci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com