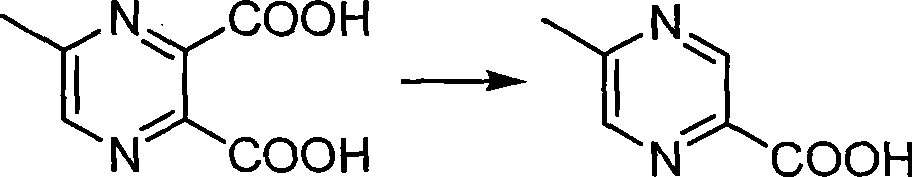
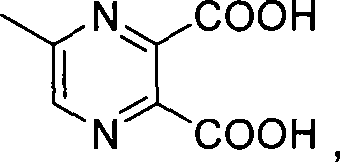
Method for preparing 5-methylprazine-2-carboxylic acid
A technology of methyl pyrazine and pyrazine dicarboxylic acid, applied in chemical instruments and methods, organic decomposition, organic chemistry and other directions, can solve the problems of high temperature, unfavorable large-scale production, harsh conditions, etc., and achieves high selectivity, green The effect of mild environmental conditions and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 80g 5-methyl-2,3-pyrazine dicarboxylic acid to a 2L four-neck bottle (1eq) and 0.96kg saturated saline (10ml / g), heated to 110oC and refluxed for 10h. When the reaction proceeds to the HPLC showing 91% conversion rate, it is cooled to room temperature, filtered with suction, and the filtrate is filtered with 1.6kg methyl ethyl ketone (25mL / g), the organic phases are combined and concentrated, cooled in an ice-water bath to crystallize, and filtered with suction to obtain the product 33.5g. HPLC purity: 98%, isomer impurity content: 1.1%, yield: 55%.
Embodiment 2
[0021] Add 36g 5-methyl-2,3-pyrazine dicarboxylic acid to a 2L four-neck bottle (1eq) and 349g saturated brine (8ml / g), heated to 108oC and refluxed for 8h. When the reaction proceeds to HPLC showing 92% conversion rate, it is cooled to room temperature, filtered with suction, and the filtrate is filtered with 524g methyl ethyl ketone (18mL / g). The organic phases are combined and concentrated, cooled in an ice-water bath to crystallize, and filtered with suction to obtain the product 15.3 g. HPLC purity: 98%, isomer impurity content: 0.7%, yield: 56.2%.
Embodiment 3
[0023] Add 54g 5-methyl-2,3-pyrazine dicarboxylic acid to a 2L four-neck bottle (1eq) and 327g saturated brine (5ml / g), heated to 105oC and refluxed for 8h. When the reaction proceeds to HPLC showing 90% conversion rate, it is cooled to room temperature, filtered with suction, the filtrate is combined with 655g methyl ethyl ketone (15mL / g), the organic phases are combined and concentrated, cooled and crystallized in an ice-water bath, and filtered with suction to obtain the product 22.5 g. HPLC purity: 98%, isomer impurity content: 2.0%, yield: 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com