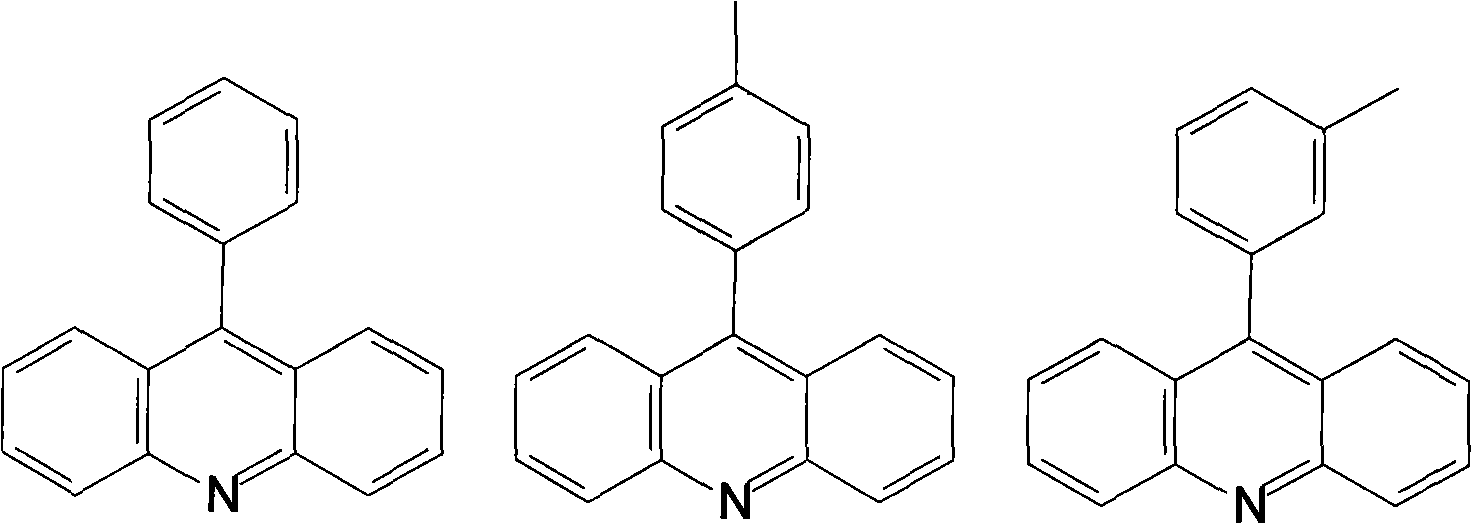
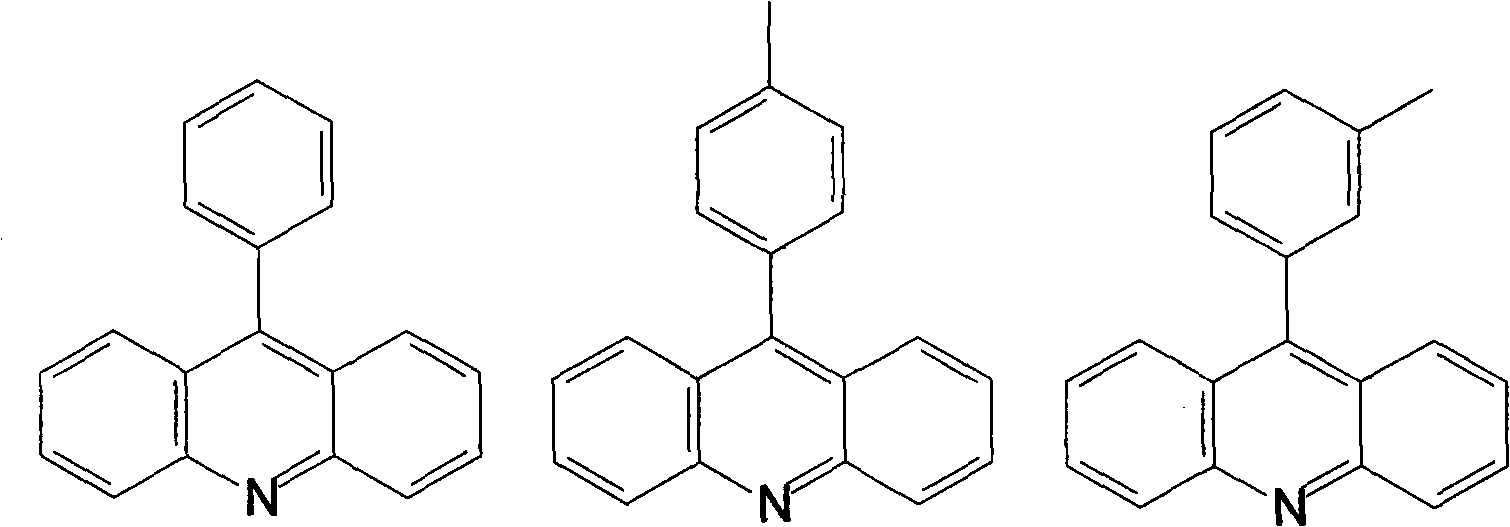
9-phenylacridine photoinitiator and preparation method thereof
A technology of phenylacridine and photoinitiator, which is applied in the direction of organic chemistry, can solve the problems of poor solubility, great influence of oxygen photocuring, and low sensitivity, and achieve the effect of stable properties, excellent sensitivity and solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment one: compound 1 synthesis process
[0026] method 1
[0027] Put 16.9g (0.1mol) of diphenylamine, 14.7g (0.12mol) of benzoic acid and 51.0g (0.375mol) of zinc chloride into a 250ml four-necked flask equipped with a water separator, heat up to 120°C, and start stirring , react at 240-250°C for 6 hours; then lower the temperature to 130-140°C, slowly add 90g of hot 33.0% sulfuric acid solution through the cooling nozzle, and stir for 30min at 100°C; when the temperature drops to about 40°C, Black sticky matter separated from the acid water appears in the bottle, pour out the acid water, add 100ml of 25% ammonia water and 120ml of toluene, stir and heat up to 60°C, after the black sticky matter is completely dissolved, separate the liquid while it is hot, and the ammonia water layer Extract with 50ml×2 toluene at 60°C, combine the toluene layers, decolorize with activated carbon, filter while hot, rotate the remaining 80ml of the toluene layer, transfer to a b...
Embodiment 2
[0032] Embodiment two: compound 2 synthesis process
[0033] Put 16.9g (0.1mol) of diphenylamine, 16.3g (0.12mol) of p-toluic acid and 51.0g (0.375mol) of zinc chloride into a 250ml four-necked flask equipped with a water separator, and heat up to 120°C , start stirring, and react for 6 hours at 240-250°C; then cool down to 130-140°C, slowly add hot 90g of 33.0% sulfuric acid solution through the condensation nozzle, and stir for 30min at 100°C; when the temperature drops to 40°C At around 10 minutes, a black sticky substance separated from the acidic water appeared in the bottle. Pour out the acidic water, add 100ml of 25% ammonia water and 120ml of toluene, stir and heat up to 60°C. The ammonia layer was extracted with 50ml×2 toluene at 60°C, the toluene layers were combined, decolorized with activated carbon, filtered while hot, and 80ml of the toluene layer was rotary evaporated, transferred to a beaker, left to stand for crystallization, filtered with a little toluene R...
Embodiment 3
[0034] Embodiment three: compound 3 synthesis process
[0035] Put 16.9g (0.1mol) of diphenylamine, 16.3g (0.12mol) of m-toluic acid and 51.0g (0.375mol) of zinc chloride into a 250ml four-necked flask equipped with a water separator, and heat up to 120°C , start stirring, and react for 6 hours at 240-250°C; then cool down to 130-140°C, slowly add hot 90g of 33.0% sulfuric acid solution through the condensation nozzle, and stir for 30min at 100°C; when the temperature drops to 40°C At around 10 minutes, a black sticky substance separated from the acidic water appeared in the bottle. Pour out the acidic water, add 100ml of 25% ammonia water and 120ml of toluene, stir and heat up to 60°C. The ammonia layer was extracted with 50ml×2 toluene at 60°C, the toluene layers were combined, decolorized with activated carbon, filtered while hot, and 80ml of the toluene layer was rotary evaporated, transferred to a beaker, left to stand for crystallization, filtered with a little toluene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com