Compound antihypertensive drug and preparation method thereof
A drug and compound technology, applied in the field of compound antihypertensive drugs and its preparation, can solve the problems of long onset time, short drug effect duration, and large blood pressure fluctuations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
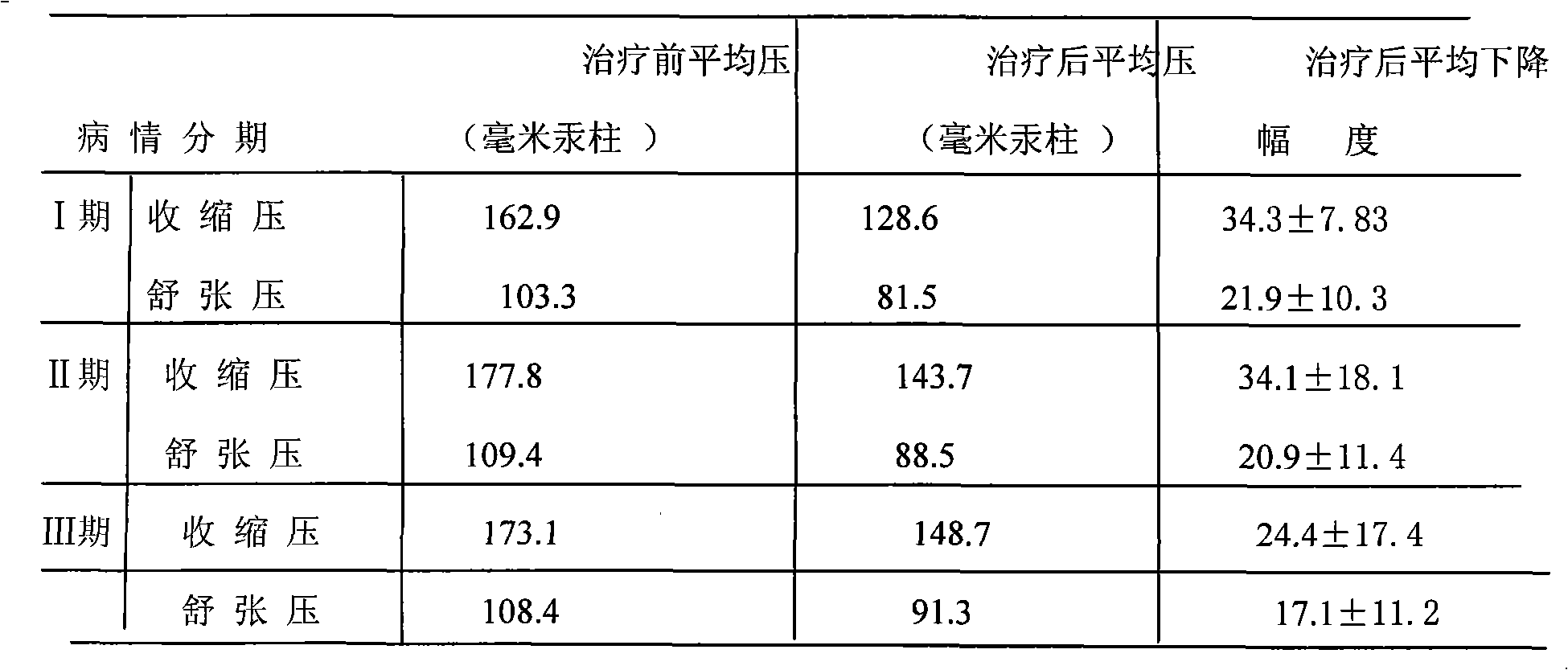
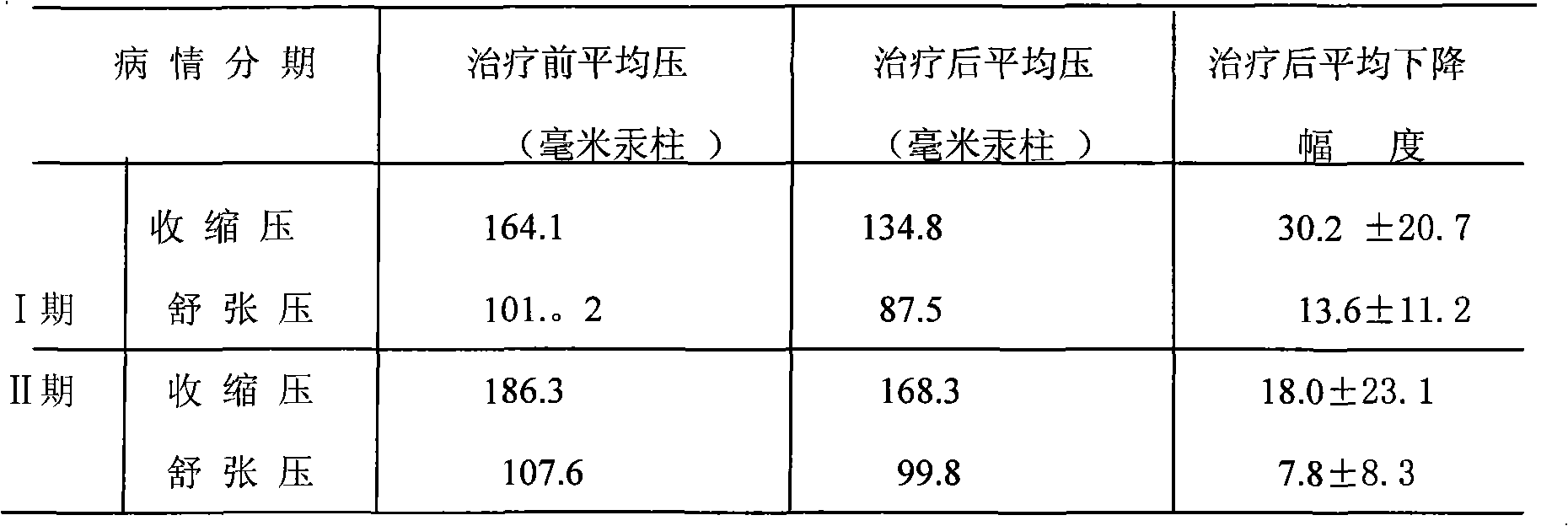
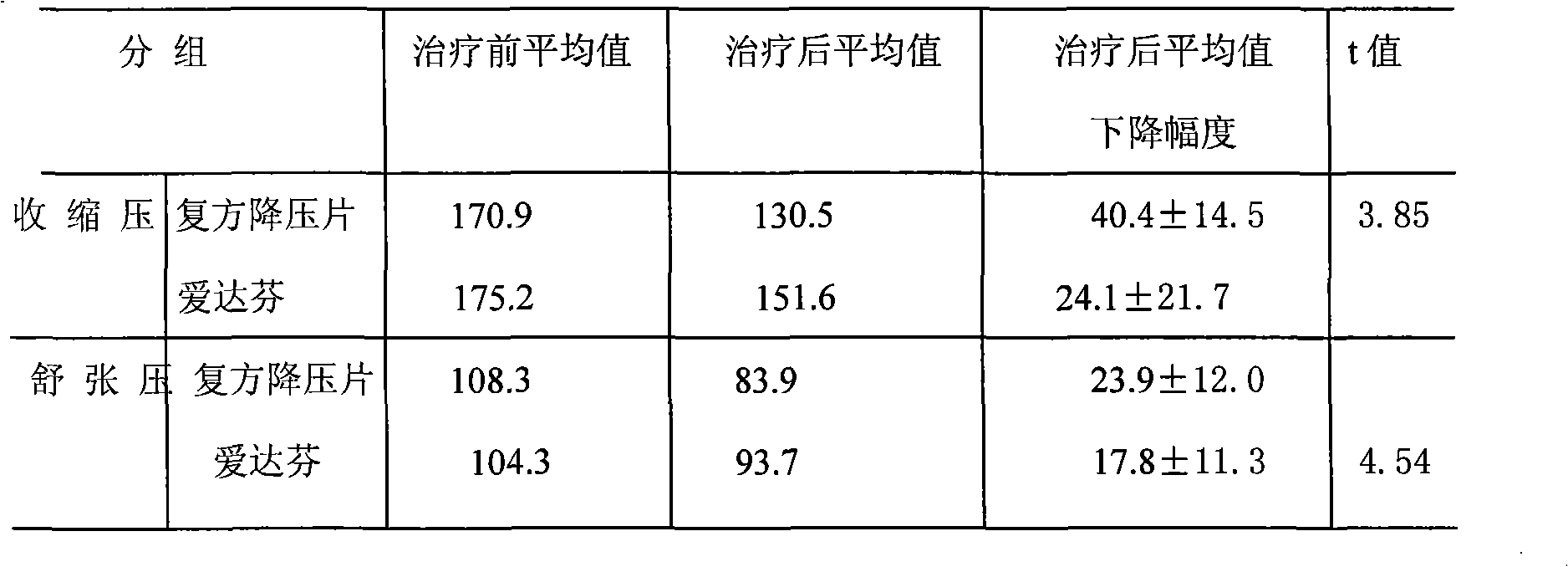
[0077] The medicine of the present invention is prepared by the following raw materials according to the weight: hydrochlorothiazide 2g, dihydralazine sulfate 1.5g, promethazine hydrochloride 2g, rutin 5g, chloroquine phosphate 2.5g, potassium chloride 30g, sulfur hydrochloride Amine g, pyridoxine hydrochloride 1g, reserpine 0.03g, magnesium trisilicate 30g, starch 3.57g, magnesium stearate 0.4g, starch slurry 20g.
[0078] It is prepared into tablets as follows:
[0079] Dihydralazine sulfate, reserpine, hydrochlorothiazide, promethazine hydrochloride, rutin, chloroquine phosphate, potassium chloride, thiamine hydrochloride, pyridoxine hydrochloride, magnesium trisilicate, and starch were added to the wet method according to the stated weight. Mix in the granulator; slowly add the starch slurry in said parts by weight into the wet granulator, the starch content in the starch slurry is 2-10%; stir for 10--60 minutes while adding to make a suitable The granules are then dried....
Embodiment 2
[0081] The medicine of the present invention is prepared by the following raw materials according to the weight: hydrochlorothiazide 2g, dihydralazine sulfate 1.5g, promethazine hydrochloride 2g, rutin 5g, chloroquine phosphate 2.5g, potassium chloride 30g, sulfur hydrochloride Amine g, pyridoxine hydrochloride 1g, reserpine 0.03g, magnesium trisilicate 30g, starch 2g, magnesium stearate 0.1g, starch slurry 10g.
[0082] Its preparation method is with embodiment 1.
Embodiment 3
[0084] The medicine of the present invention is prepared by the following raw materials according to the weight: hydrochlorothiazide 2g, dihydralazine sulfate 1.5g, promethazine hydrochloride 2g, rutin 5g, chloroquine phosphate 2.5g, potassium chloride 30g, sulfur hydrochloride Amine g, pyridoxine hydrochloride 1g, reserpine 0.03g, magnesium trisilicate 30g, starch 5g, magnesium stearate 0.8g, starch slurry 30g.
[0085] Its preparation method is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com