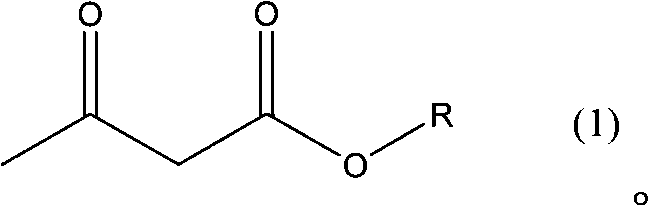
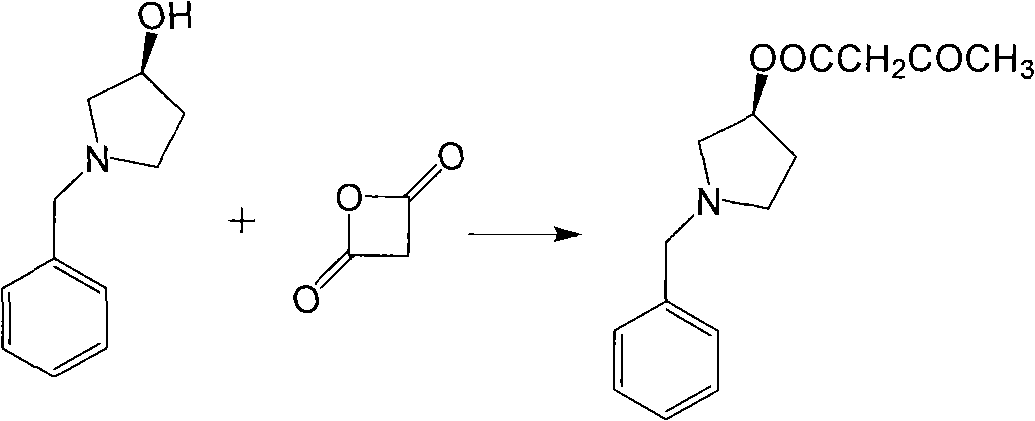
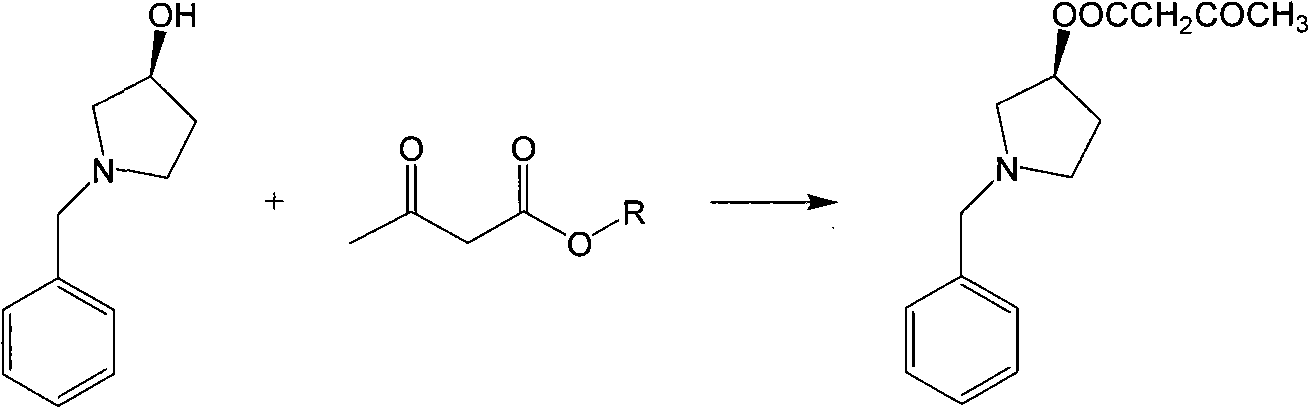
New preparation method of intermediate product of barnidipine hydrochloride
A technology of barnidipine hydrochloride and an intermediate, which is applied in the field of preparation of a barnidipine hydrochloride intermediate-N-benzyl-3-acetoethoxy pyrrolidine ester, can solve the problem that diketene has a low boiling point, is not suitable for industrial production, and is not suitable for industrial production. Dangerous issues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] In a 1000ml three-necked flask, add 1 mol of methyl acetoacetate, 1 mol of (S)-N-benzyl-3-hydroxypyrrolidine, add 0.1 mol of catalyst p-toluenesulfonic acid, heat and reflux for 12 hours, stop the reaction, and remove by distillation under reduced pressure. Solvent, add appropriate amount of water and ethyl acetate for extraction, separate and dry to obtain (S)-N-benzyl-3-acetoethoxypyrrolidinate. The product was determined to be 262.1 (M+H) by electrospray ionization mass spectrometry (ESI-MS). + ) (theoretical molecular weight is 261.14), b.p. is 130-135°C / 0.1mmHg (literature is 130-135°C / 0.1mmHg).
Embodiment 2
[0015] In a 1000ml three-neck flask, add 1 mol of methyl acetoacetate, 5 mol of (S)-N-benzyl-3-hydroxypyrrolidine, add 0.1 mol of benzenesulfonic acid as a catalyst, heat to reflux for 24 hours, stop the reaction, and distill off the solvent under reduced pressure , adding an appropriate amount of water and ethyl acetate for extraction, separation, and drying to obtain (S)-N-benzyl-3-acetoethoxypyrrolidinyl ester. The product was measured by electrospray ionization mass spectrometry (ESI-MS) at 262.1 (M+H + ) (theoretical molecular weight is 261.14), b.p. is 130-135°C / 0.1mmHg (literature is 130-135°C / 0.1mmHg).
Embodiment 3
[0017] In a 1000ml three-neck flask, add 3 mol of ethyl acetoacetate, 1 mol of (S)-N-benzyl-3-hydroxypyrrolidine, add 0.1 mol of catalyst p-toluenesulfonic acid, heat and reflux for 6 hours, stop the reaction, and remove by distillation under reduced pressure. Solvent, add appropriate amount of water and ethyl acetate for extraction, separate and dry to obtain (S)-N-benzyl-3-acetoethoxypyrrolidinate. The product was measured by electrospray ionization mass spectrometry (ESI-MS) at 262.1 (M+H + ) (theoretical molecular weight is 261.14), b.p. is 130-135°C / 0.1mmHg (literature is 130-135°C / 0.1mmHg).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com