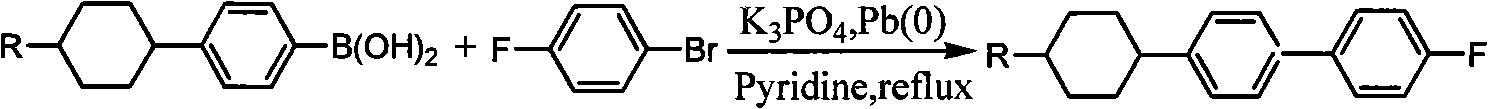Method for synthesizing fluorine-containing antiform alkyl cyclohexyl biphenyl single liquid crystal
A technology based on biphenyls and liquid crystal monomers, applied in the direction of liquid crystal materials, chemical instruments and methods, preparation of halogenated hydrocarbons, etc., can solve the problems of excessive discharge of three wastes, rising costs, and inability to produce liquid crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
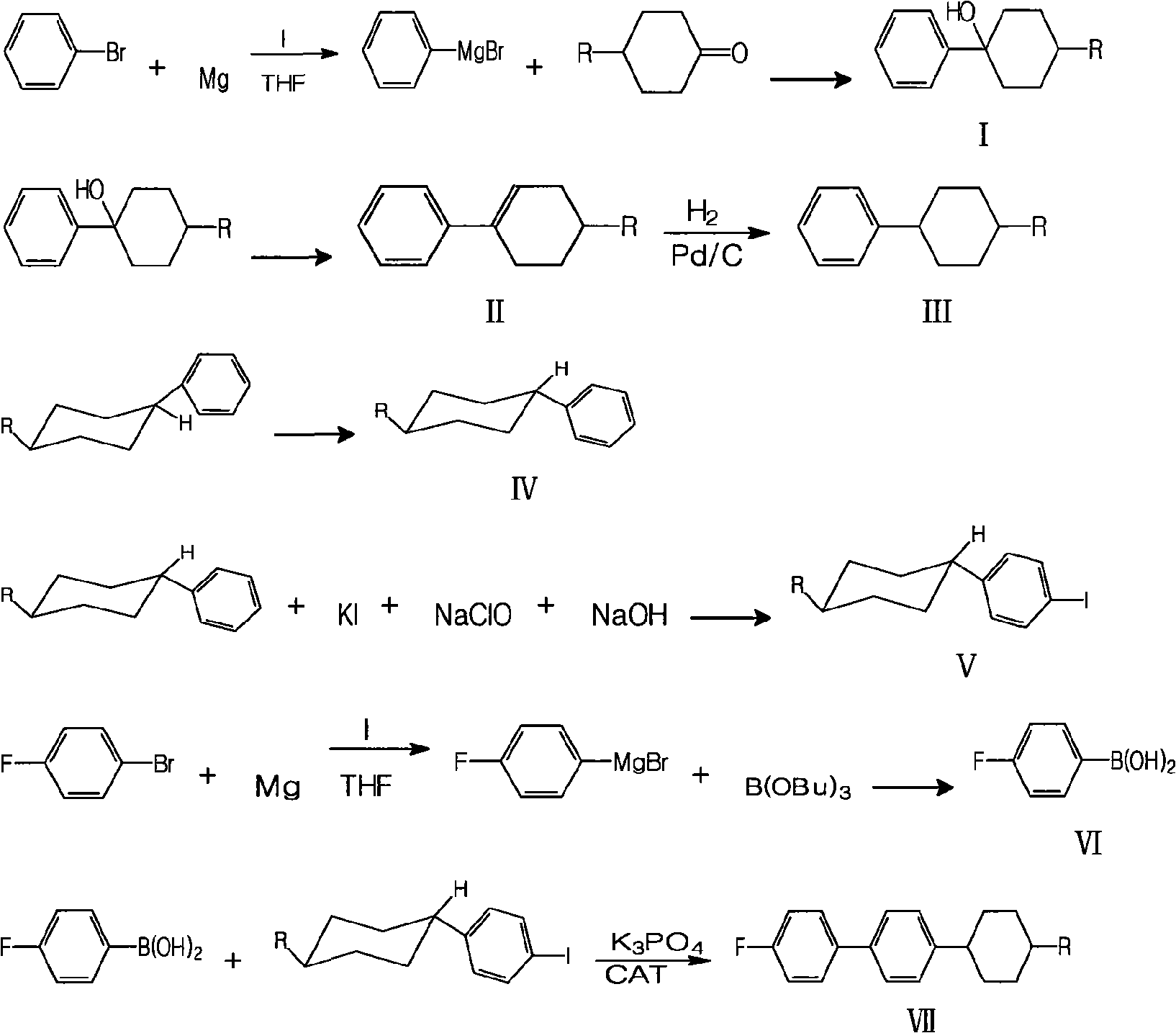
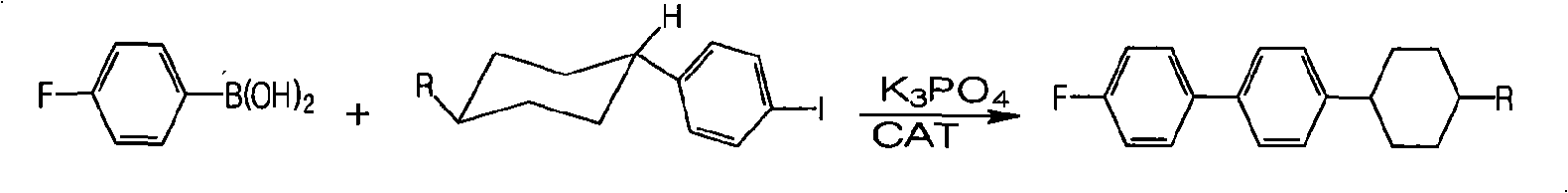
[0049] Example, a method for synthesizing fluorine-containing trans-alkylcyclohexylbiphenyl liquid crystal monomers is to select bromobenzene and magnesium for Grignard reaction, then couple with alkylcyclohexanone and hydrolyze, then dehydrate, Synthesis of alkyl cyclohexyl iodobenzene by hydrogenation, transformation and iodination, Grignard reaction between p-fluorobromobenzene and magnesium, then low-temperature coupling to synthesize p-fluorophenylboronic acid, and finally the target compound was synthesized by coupling reaction, which is the synthesis of The route is as follows:
[0050]
[0051]
[0052] Where I is 4-alkyl-1-phenylcyclohexanol, II is 4-alkyl-1-phenylcyclohexene, III is alkylcyclohexylbenzene, IV is trans-alkylcyclohexylbenzene, V is trans-alkylcyclohexyl iodobenzene, VI is p-fluorophenylboronic acid, VII is 4-(4-alkylcyclohexyl)-4',-fluoro-1-1',-biphenyl, R is represented by alkane Base, representing ethyl, propyl, butyl, pentyl respectively; Thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com