Aqueous solution and method of prolonging life of residual chlorine in aqueous solution
A technology of aqueous solution and perchloric acid, applied in the field of aqueous solution, can solve the problems of shortened service life of hypochlorous acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Make KClO 3 Dissolved in pure water.
[0104] In the KClO 3 Add citric acid to the aqueous solution. Thus, the pH was adjusted to about 4.
[0105] Measure the KClO of this pH4 3 The residual chlorine concentration of the aqueous solution.
[0106] In addition, the KClO at pH 4 above 3 Add H to the aqueous solution 2 O 2 Aqueous solution, determine the residual chlorine concentration.
[0107] The results of this measurement (measured by the KI method) are shown in Table-1 below.
[0108] [Table 1]
[0109] Table 1
[0110] Elapsed time Concentration (ppm)
[0111] Potassium chlorate 125 125 125 125 125 125
[0112] Hydrogen perchloride 0 30 60 120 240 480
[0113] Residual chlorine 0 days 0 5 25 30 30 125
[0114] 7 days 0 200 250 300 300 350
[0115] 14 days 0 200 250 300 300 350
[0116] 30 days 0 200 250 300 300 350
[0117] It can be seen from the table-1 that by containing H 2 O 2 , Even after days, KClO 3 The residual chlorine conc...
Embodiment 2
[0120] Make KClO 3 Dissolved in pure water.
[0121] In the KClO 3 Add citric acid to the aqueous solution. Thus, the pH was adjusted to about 4.
[0122] In the KClO 3 NaClO is further added to the aqueous solution.
[0123] Then, the residual chlorine concentration is measured. The result is not significantly different from the case of the NaClO aqueous solution.
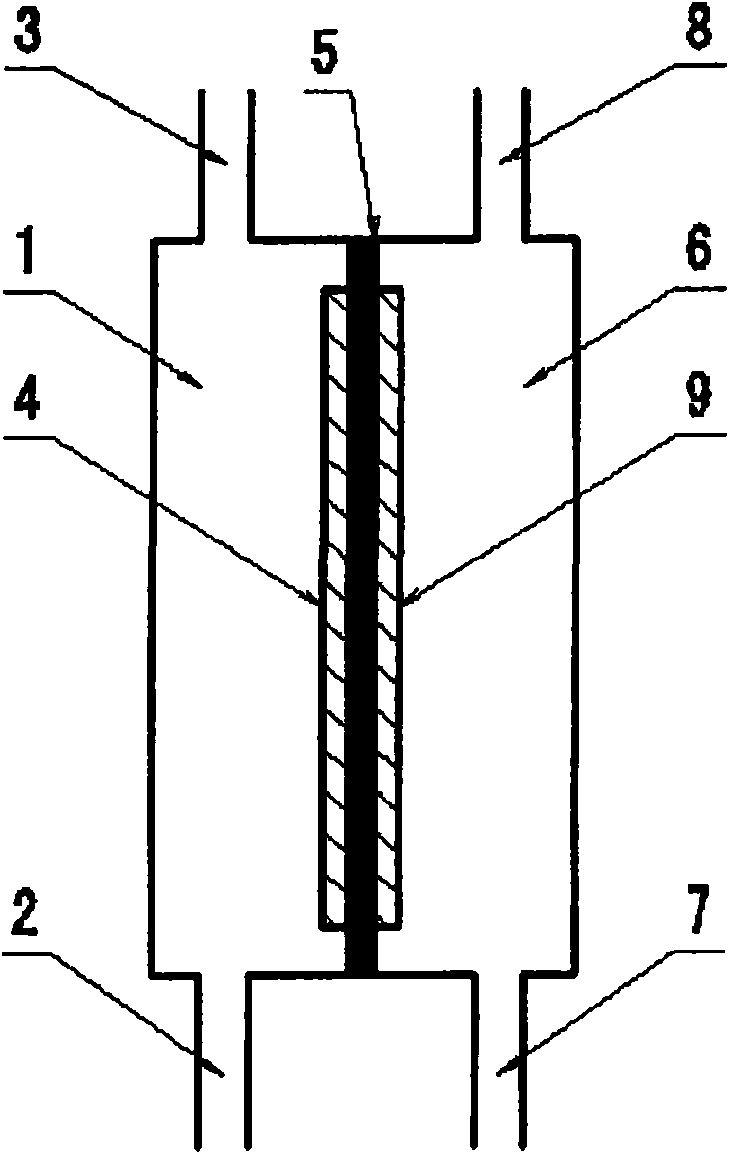
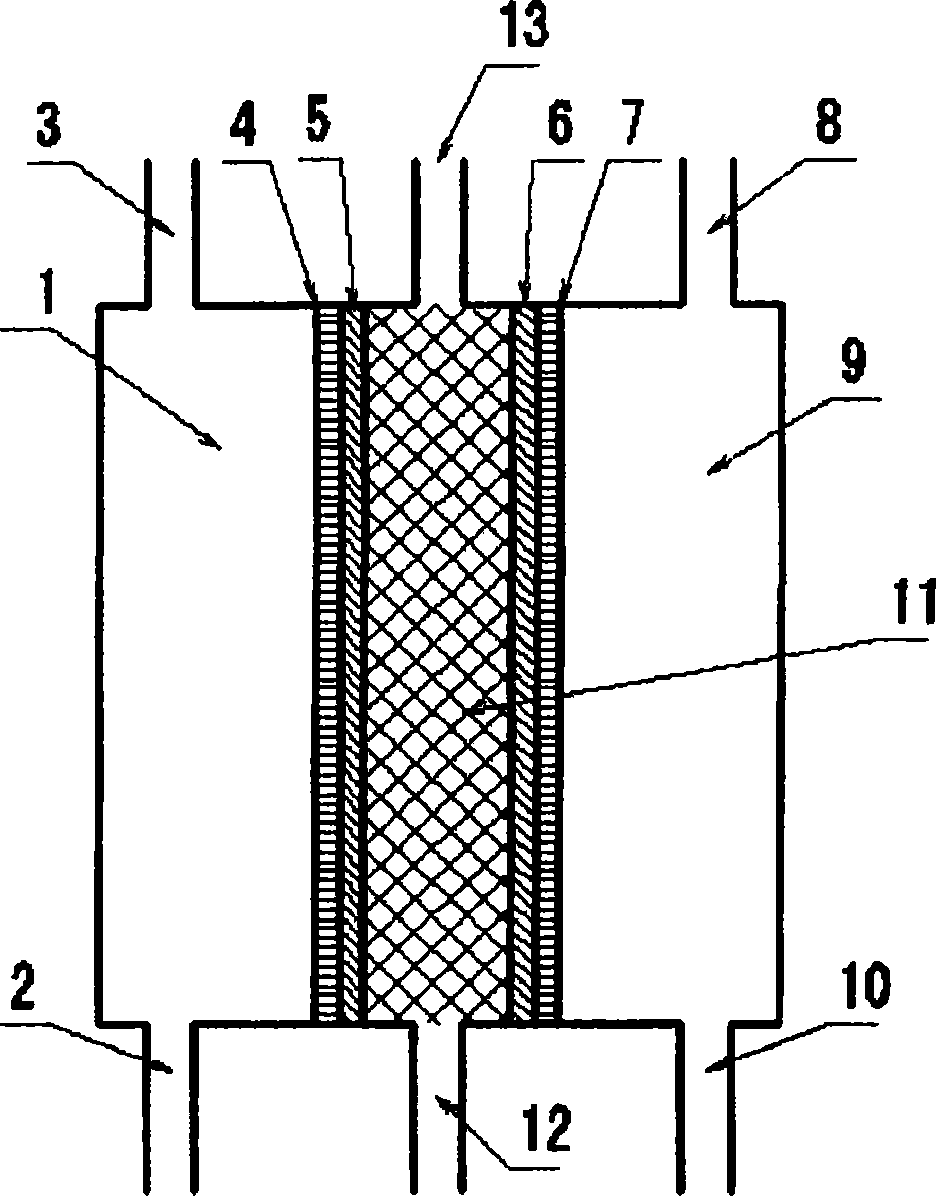
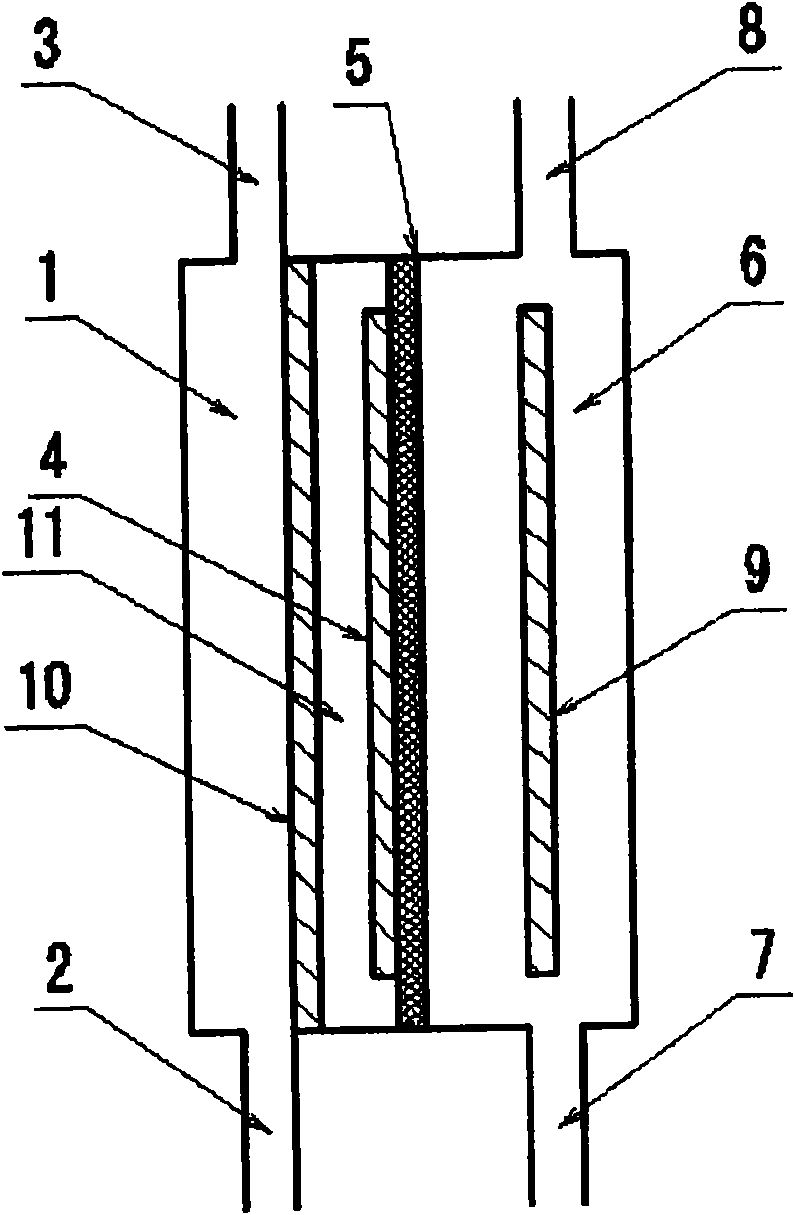
[0124] Then, use figure 1 The anode electrolyzed water produced in the illustrated two-compartment electrolytic cell replaces the above-mentioned pure water. The two-chamber electrolytic cell is as follows: use 80 mesh platinum electrode (the size of the electrode is 80mm×60mm) as the positive electrode, use the titanium electrode (the size of the electrode is 80mm×60mm) as the negative electrode, and use the fluorine-based cation exchange membrane as the The diaphragm that separates the anode and cathode compartments. Then, pure water is supplied to the cathode chamber and the anode chamber.
[0125] 80 ppm NaClO wa...
Embodiment 3
[0129] Dissolve 40ppm NaClO and 100ppm KClO in the anode electrolyzed water of Example 2 2 . Further, citric acid was added to adjust the pH to about 6.
[0130] Determine the content of NaClO and KCIO 2 The residual chlorine concentration of the aqueous solution (measured by the KI method), the results are shown in Figure 8 .
[0131] By this Figure 8 It can be seen that the long-term high content of NaClO and KClO 2 The residual chlorine concentration of the aqueous solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com