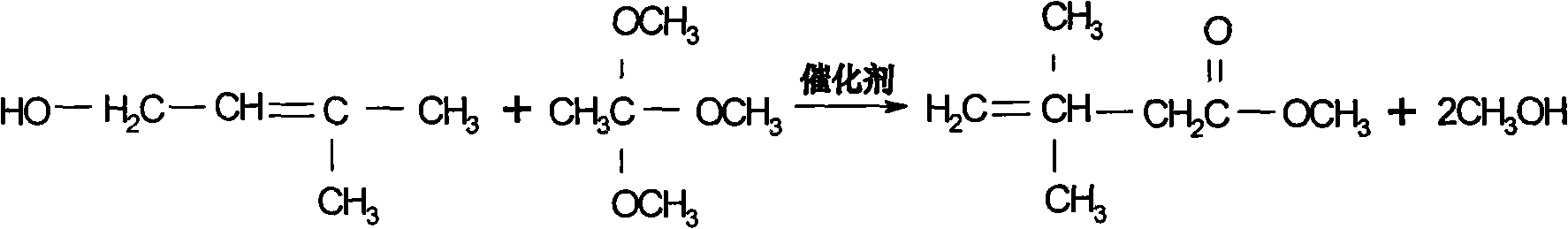
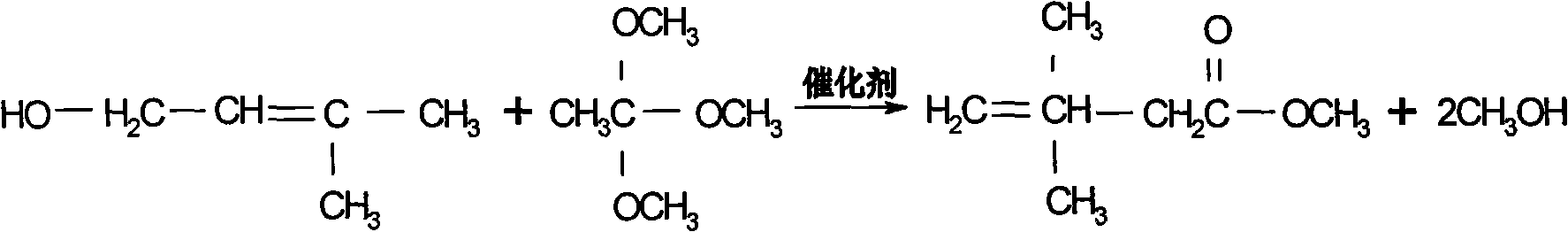
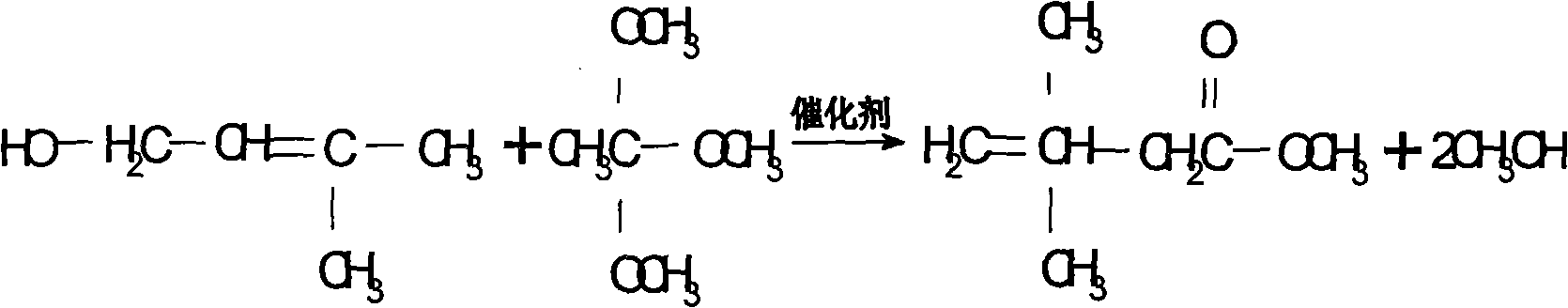
Preparation process of 3,3-dimethyl-4-pentenoic acid methyl ester
A technology for the preparation of methyl pyretinate, which is applied in the field of preparation of methyl pyretinate, can solve the problems of the loss of pyretinic acid, reduce the yield, and high production cost, and achieve the effect of increasing the yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The molar ratio of reactants is trimethyl orthoacetate: isopentenol: catalyst = 1: 0.3: 0.02, add a certain amount of trimethyl orthoacetate, pump in the catalyst phosphoric acid \ sodium alkoxide and raise the temperature to 85 °C and start to drop isoamyl For pentenol, the dropping time is controlled within 5 hours. After the dropping is completed, the temperature of the kettle rises to about 95°C, and at the same time, it continues to receive the fore distillation with a top temperature <45°C. The reaction time is 3 hours at 90°C, and then the temperature of the kettle is gradually raised to about 100°C in 5 hours. In 12 hours, speed up the collection of the second distillate, which takes about 6 hours. When the second distillate is received, the temperature of the kettle is about 120~125°C. After the reaction is completed, the temperature is lowered, and then the material is fed to the crude ester crude product storage tank for rectification. The molar yield that ob...
Embodiment 2
[0025] The molar ratio of the reactants is trimethyl orthoacetate: isopentenol: catalyst = 1:0.5:0.03, add a certain amount of trimethyl orthoacetate, pump in the catalyst phosphoric acid\sodium alkoxide and raise the temperature to 80°C to start adding isoamyl For pentenol, the dropping time is controlled within 6 hours. After the dropping is completed, the temperature of the kettle rises to about 100°C, and at the same time, it continues to receive the fore distillation with a top temperature <45°C. The reaction time is 2 hours at 95°C, and then the temperature of the kettle is gradually raised to about 105°C in 6 hours. After holding for 4 hours, the second fraction is received, the top temperature is gradually raised to about 100°C, and the temperature of the kettle is gradually raised to about 135°C. Time control In 12 hours, speed up the collection of the second distillate, which takes about 6 hours. When the second distillate is received, the temperature of the kettle is...
Embodiment 3
[0027] The molar ratio of the reactants is trimethyl orthoacetate: isopentenol: catalyst = 1:0.4:0.05, add a certain amount of trimethyl orthoacetate, pump in the catalyst phosphoric acid\sodium alkoxide and raise the temperature to 82°C and start to drop the isoamyl For pentenol, the dropwise addition time is controlled within 4 hours. After the dropwise addition, the temperature of the kettle rises to 95°C, and at the same time, it continues to receive the fore distillation with a top temperature <45°C. The reaction time at 100°C is 4 hours, and then the temperature of the kettle is gradually raised to about 100°C in 5 hours. After holding for 5 hours, the second fraction is received, the top temperature is gradually raised to about 100°C, and the temperature of the kettle is gradually raised to about 135°C. Time control In 12 hours, speed up the collection of the second distillate, which takes about 6 hours. When the second distillate is received, the temperature of the kett...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com