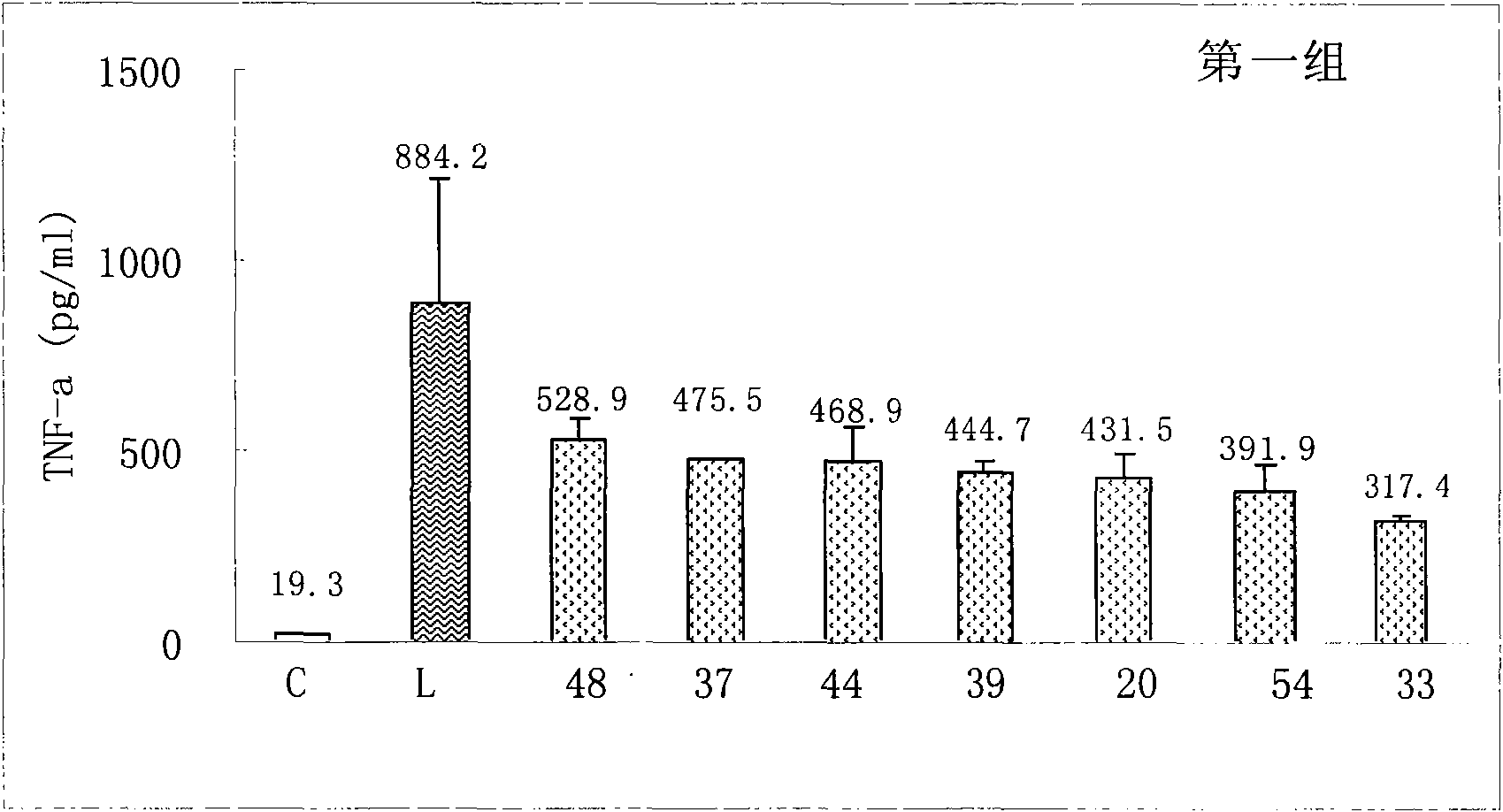
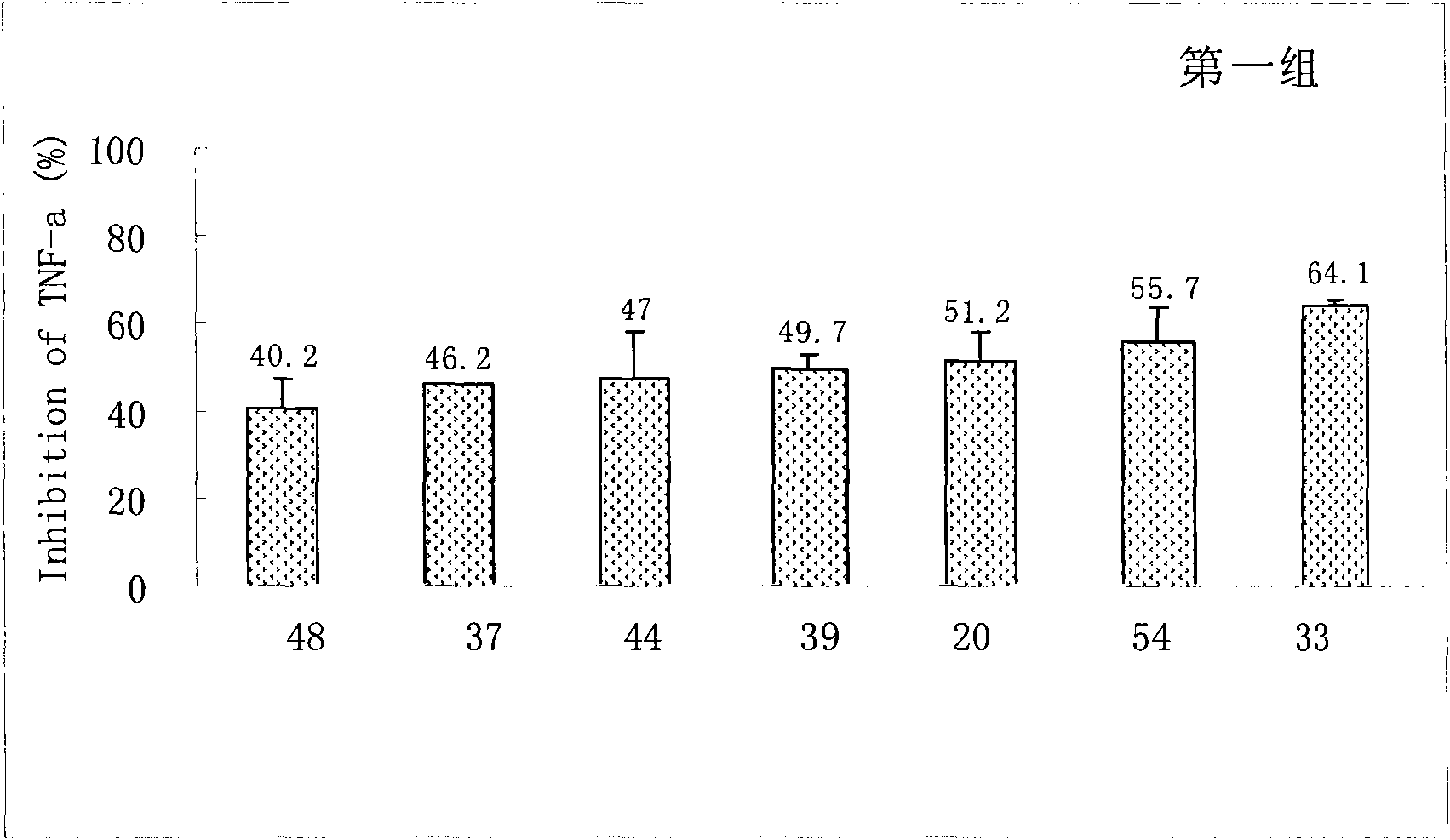
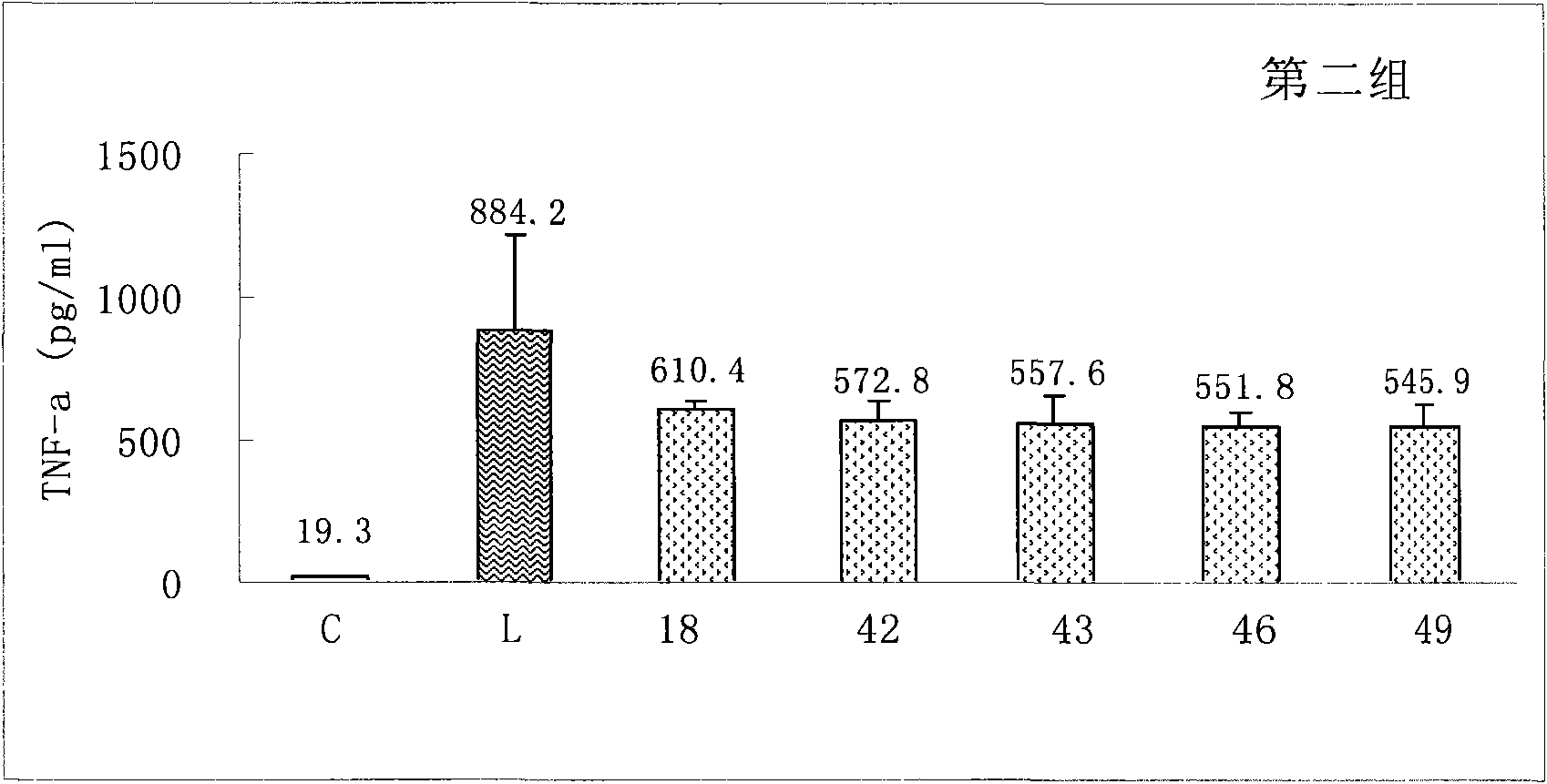
Sinomenine derivatives and preparation and uses thereof
A technology of sinomenine and compound, which is applied in the field of preparation and application of sinomenine derivatives, and can solve problems such as low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0050] The preparation of example 1 17-demethylbenzyloxybenzyloxy sinomenine
[0051] The first step: preparation of benzyloxybenzyloxysinomenine (Hitotsuyanagi, Y.; Nishimura, K.; Ikuta, H.; Takeya, K.; Itokawa, H.J.Org.Chem., 1995, 60 (14), 4549-58.)
[0052]
[0053] Sinomenine (1.65g, 5mmol), PPh 3 (3.93g, 15mmol), BnOBnOH (3.17g, mmol) and anhydrous THF (50ml) were mixed in a 25ml 4-neck round bottom flask equipped with a magnetic stirring ladle and stirred. The reaction mixture was cooled with ice, and DEAD (2.61 g, 15 mmol) was slowly added dropwise over 30 minutes. After the addition, it was stirred at room temperature for 12 hours. The THF was spun off to leave a residue, which was purified through a silica gel column (ethyl acetate, then methanol) to obtain a flesh-colored solid. A white powder (2.6 g) was obtained by recrystallization of isopropyl ether. mp 72°C; [α] 25 D -69.0° (c=0.28CHCl 3 ); 1 HNMR (300MHz, CDCl 3 )δ: 7.56 (d, 2H, J = 8.6Hz.), 7.48-7....
example 2
[0058] Example 2 Preparation of 17-propanesulfonylsinomenine
[0059]
[0060] first step:
[0061] In a solution of 17-desmethylbenzyloxybenzyloxysinomenine (100mg) and dichloromethane (10ml) containing triethylamine, slowly add propanesulfone containing dichloromethane (5ml) under stirring acid chloride (50 mg) solution. Stirred for 5 minutes, the reaction mixture was evaporated under reduced pressure to obtain a residue, and the residue was refined with a silica gel column (ethyl acetate / petroleum ether at a volume ratio of 1:2) to obtain 17-propanesulfonylbenzyloxybenzyloxy ivy alkali. [α] 25 D +210.5°(c=0.06CHCl 3 ); 1 HNMR (300MHz, DCCl 3 )δ: 7.56(d, 1H, J=8.7Hz), 7.48-7.34(m, 4H), 7.03(d, 1H, J=8.7Hz), 6.81(d, 1H, J=8.7Hz), 6.78( d, 1H, J=8.7Hz), 5.47(s, 1H), 5.27(d, 1H, J=10.5Hz), 5.11(s, 1H), 5.01(d, 1H, J=10.5Hz), 4.37( s, 1H), 4.21(d, 1H, J=15.9Hz), 3.85(s, 3H), 3.53(s, 3H), 3.52-3.44(m, 1H), 3.28-3.20(m, 1H), 2.97 -2.85(m, 4H), 2.74-2.55(m, 1H), 2.47(d...
example 3
[0065] Example 3 Preparation of 17-p-toluenesulfonyl sinomenine
[0066]
[0067] first step:
[0068] In a mixture of 17-desmethylbenzyloxybenzyloxysinomenine (100mg) and triethylamine (0.1ml) in dichloromethane (10ml), add 5ml of dichloromethane containing 30mg of p-toluenesulfonyl chloride dropwise methane solution. Mix and stir at room temperature for 10 minutes, and then evaporate under reduced pressure to obtain a crude product of 17-p-toluenesulfonylbenzyloxybenzyloxysinomenine, which can be directly used in the next reaction without purification.
[0069]
[0070] Step two:
[0071] 100mg of 17-p-toluenesulfonylbenzyloxybenzyloxysinomenine obtained in the first step of this example, as shown in the second step in Example 2, after adding 10ml of 5% TFA / DCM solution, can produce 50 mg of 17-p-toluenesulfonyl sinomenine. mp 141.0-143.0°C; [α] 10 D +92.4°(c=0.49CHCl 3 ); 1 HNMR (300MHz, DCCl 3 )δ: 7.74(d, 2H, J=7.6Hz.), 7.33(d, 2H, J=7.6Hz.), 6.62(d, 1H, J=8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com