Method for synthesizing novel tetracyclic diterpene compound from stevioside
A technology for tetracyclic diterpenoids and compounds, which is applied in the field of synthesizing novel tetracyclic diterpenoids from stevioside, and can solve the problem of less research on structural synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example 2
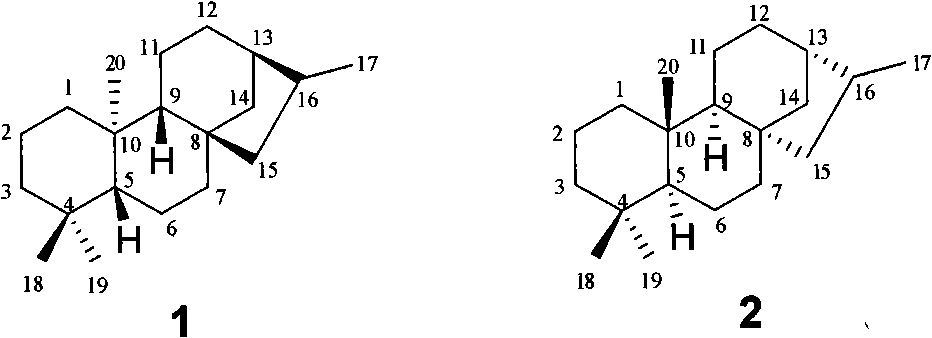
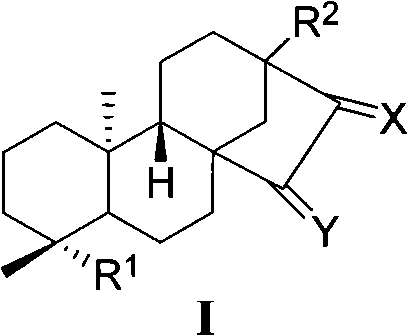
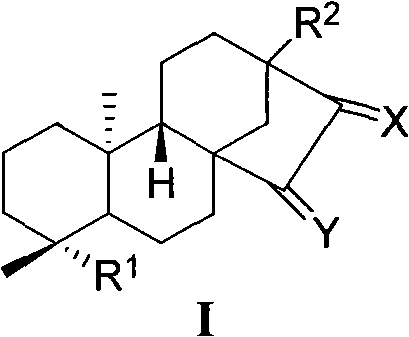
[0062] En-13,15-dihydroxykaurene-19-acid (15-hydroxysteviol) (I-a)
[0063] 0.48g SeO 2 , 1.76 mL t-BuOOH was dissolved in 45 mL THF. After stirring for a while, 2.00 g of steviol (I-1) dissolved in 50 mL of THF was added. Reaction at room temperature for 20h. Saturated brine dissolved with a small amount of sodium bisulfite was added to the reaction system, extracted with ethyl acetate, and the extract was washed with saturated brine. After concentrating the extract, the crude product can be directly dropped into the next step reaction. If purification is required, column chromatography (ethyl acetate:petroleum ether (V / V)=2.5:1 and the addition of acetic acid with a volume of 0.3% of the total volume of acetic acid and petroleum ether) can give 1.25g of pure white solid. 59.5%. ESI-MS: 333 [M-H] - .
specific example 3
[0065] En-13-Hydroxy-15-carbonylkaurene-19-acid (15-carbonyl steviol) (I-1)
[0066] 0.50g 15-hydroxysteviol (I-a), 0.80g PDC were dissolved in 2mL DMF. React overnight at room temperature. Add saturated brine to the reaction system, extract with ethyl acetate, wash the extract with saturated brine, and dry over anhydrous sodium sulfate. After concentrating the extract, the crude product was subjected to column chromatography (ethyl acetate:petroleum ether (V / V)=1:1 and 0.3% acetic acid was added to the total volume of ethyl acetate and petroleum ether). Obtained 0.36 g of pure white solid, yield: 71.5%. m.p.236-238°C.
[0067] IR(KBr)v cm -1 : 3511, 3457, 3419, 2997, 2946, 2865, 1692, 1648, 1459, 1396, 1338, 1242, 1189, 1156, 1095, 1046. 1 H NMR (400MHz, DMSO-d 6 , δ, ppm): 11.99 (s, 1H, -COOH), 5.82 (s, 1H, 17-H), 5.35 (s, 1H, 17-H), 2.32 (d, 1H J=12Hz, 14-Hα ), 1.14(s, 3H, 18-C H 3 ), 0.95(s, 3H, 20-C H 3 ).ES1-MS: 331[M-H] - .
[0068] Wherein PDC is prepared ...
specific example 4
[0070] En-13-Hydroxy-15-Carbonyl-Kurene-19-n-Propionamide (I-2)
[0071] Dissolve 0.500g of 15-hydroxy steviol (I-a) in 10mL of anhydrous THF and 3mL of anhydrous DMF, add 0.402g of DCC, 0.298g, HoBt, stir at room temperature for 2h, then add 0.432mL of n-propylamine. Heated to reflux for 8h. The reaction solution was left to cool and then filtered, 50 mL of water was added to the filtrate, extracted twice with 30 mL of ethyl acetate, the extracts were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain the crude product enantio-13,15 - Dihydroxykaurene-19-propionamide 0.510 g.
[0072] Dissolve the above crude product in 6 mL of DMF, add 0.78 g of PDC, react at room temperature for 12 hours, add the reaction solution to 30 ml of water, extract three times with 20 ml of ethyl acetate, combine the extracts, wash with saturated saline and evaporate the solvent to obtain enantio-13-hydroxy 0.49 g of the crude prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com