Selenium-containing active polypeptide, preparing method and application thereof
An active polypeptide and an active technology, which is applied in the application field of the polypeptide in the preparation of drugs, can solve the problems of disulfide bond breakage or mismatch, high cost, difficult process, etc., improve the correct folding rate and reduce the dosage , good effect of peptide stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] [Example 1] [Sec 1,16 ] Synthesis of MVIIA (Pseudo-ω-MVIIA Peptide A)
[0052] (1) Solid phase synthesis: According to the pseudo ω-MVIIA peptide [Sec 1,16 ] MVIIA amino acid sequence, using Boc solid-phase chemical synthesis method, using HBTU as coupling reagent and Boc-amino acid (Sigma), MBHA (4-methylbenzhy-drylamine) resin (Advanced Technology & Industrial company) to carry out artificial synthesis. Synthesis of Boc-Sec(MeBzl)-OH: 5g of selenocysteine (Sigma) was added to 20ml of 1M NaOH solution, stirred at 0°C, and 20ml of 0.8M sodium borohydride solution was added at the same time for about 40 minutes. Adjust the pH to 7.0 with glacial acetic acid. Dissolve 1.5g of α-bromoxylene in 20ml of ethanol, add dropwise to the previous reaction solution, and react at 0°C for 2.5 hours. Adjust the pH to 2 with 6M hydrochloric acid to obtain 4-benzyl-L-selenocysteine as a white precipitate. 4-Benzyl-L-selenocysteine 4g with K 2 CO 3 Mix 4.5g and dissolve in 30...
Embodiment 2
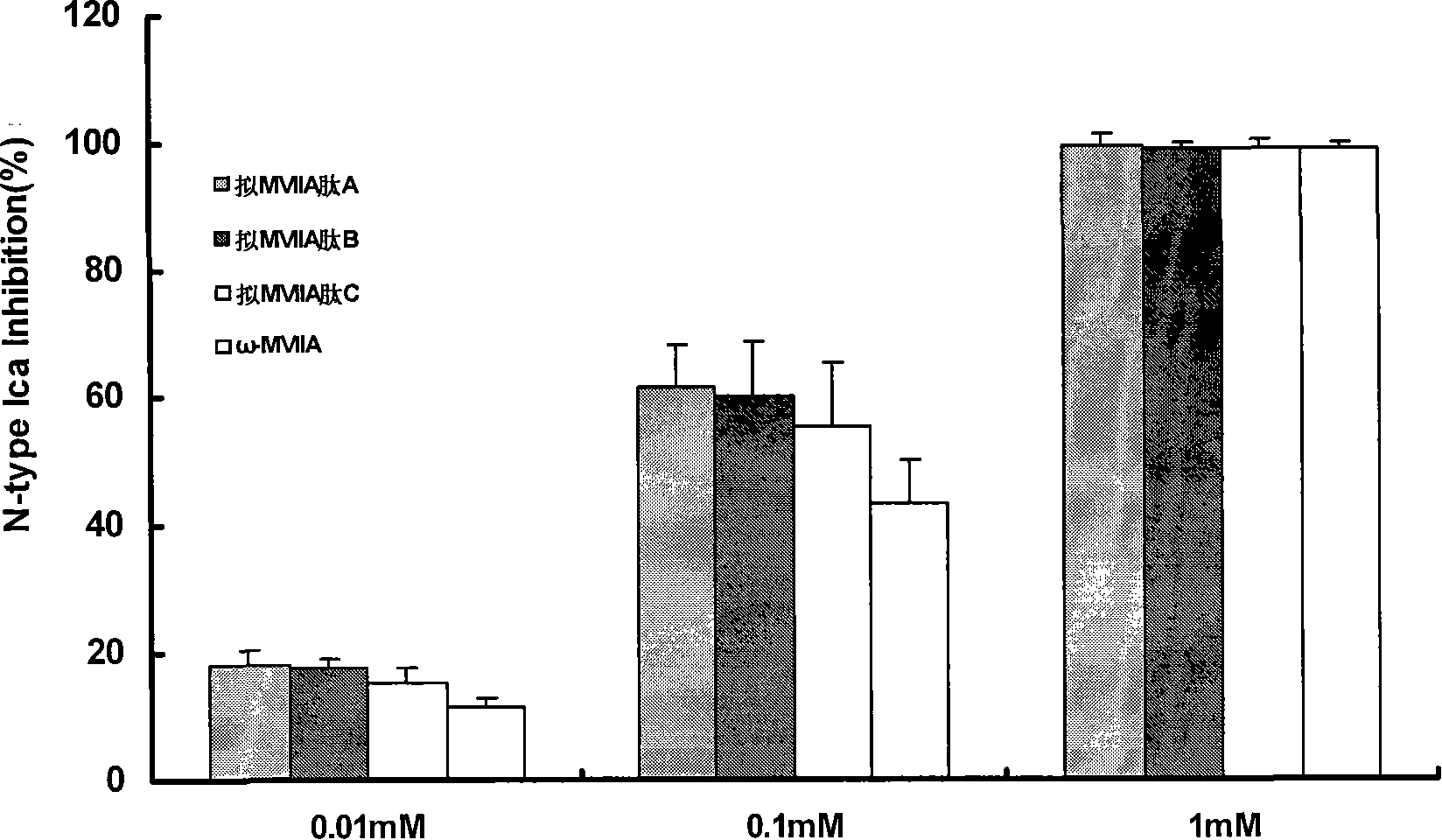
[0054] [Example 2] Synthetic [Sec 8,20 ], [Sec 15,25 ], [Sec 1,16,8,20 ], [Sec 1,16,15,25 ], [Sec 8,20,15,25 ], [Sec 1,16,8,20,15,25 ] MVIIA (pseudo-omega-MVIIA peptides B, C, D, E, F, G)
[0055] According to the pseudo ω-MVIIA peptide [Sec 8,20 ], [Sec 15,25 ], [Sec 1,16,8,20 ], [Sec 1,16,15,25 ], [Sec 8,20,15,25 ], [Sec 1,16,8,20,15,25 ] MVIIA sequence, prepare the pseudo ω-MVIIA peptide according to the steps of synthesis, bond formation and folding described in Example 1.
Embodiment 3
[0056] [Example 3] Pseudo-ω-MVIIA peptide molecular weight determination
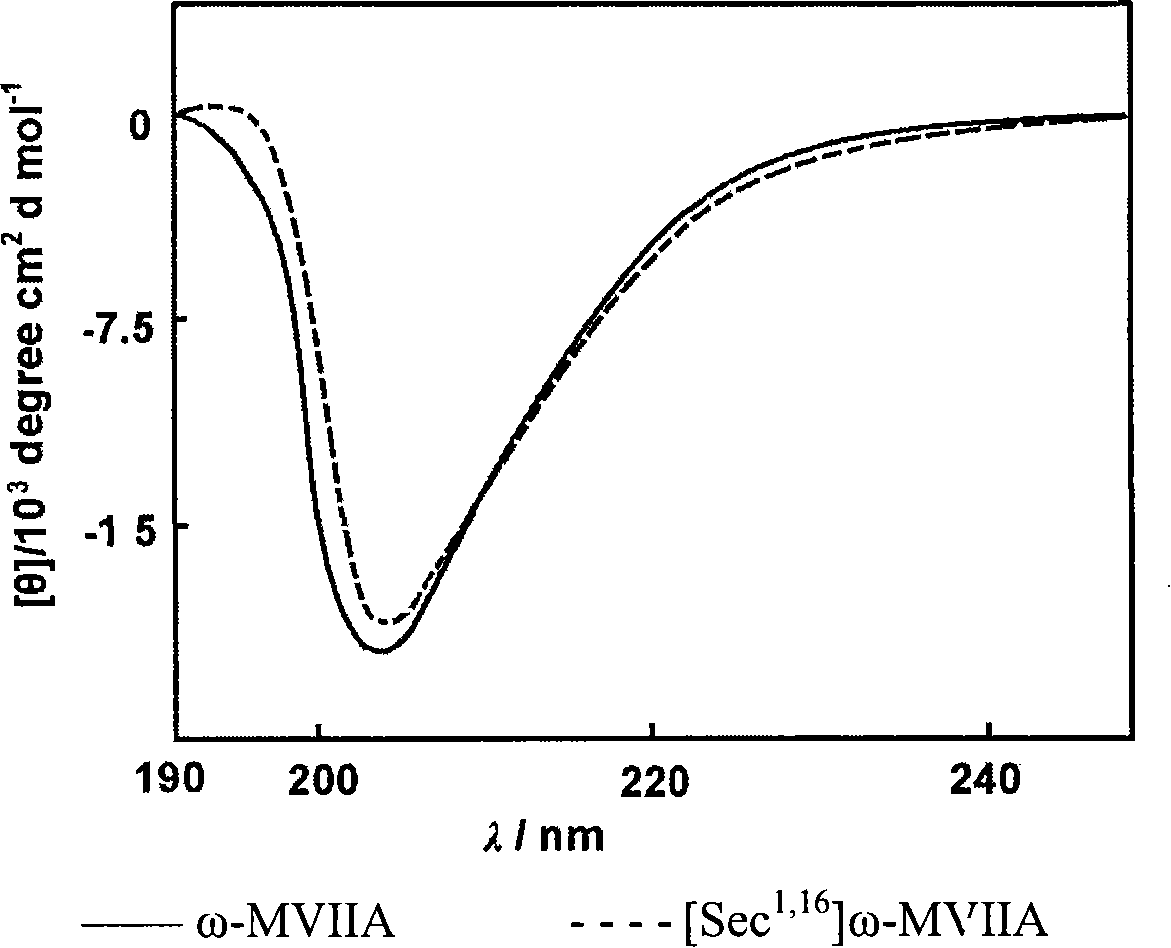
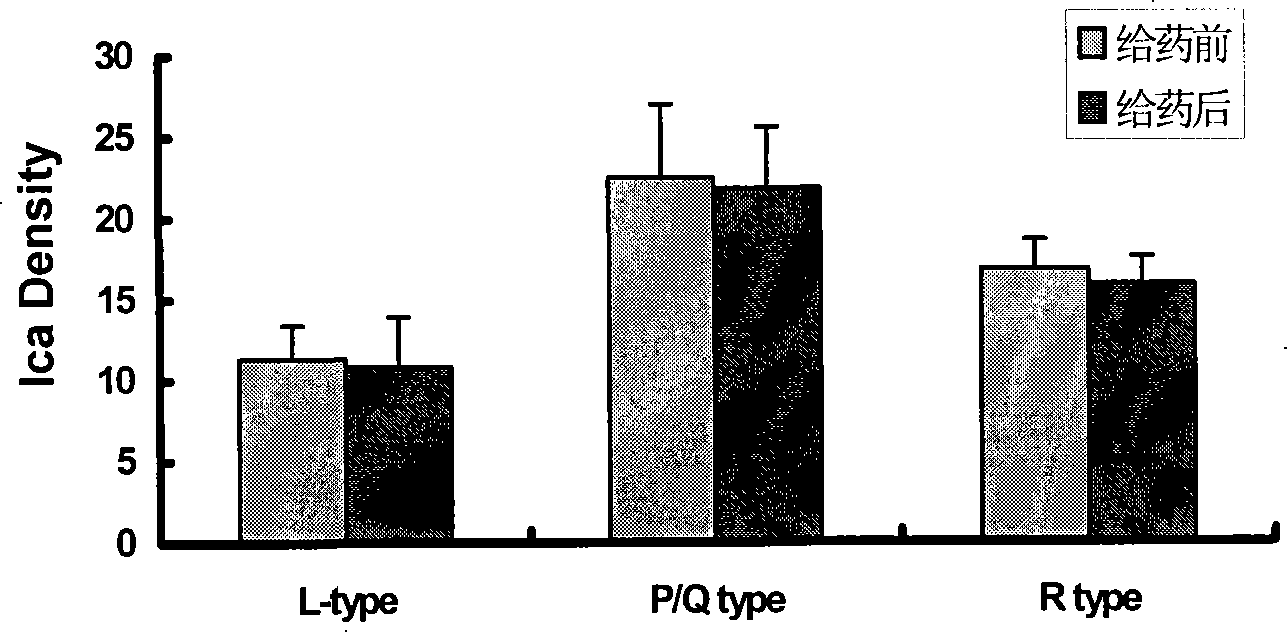
[0057] The quasi-ω-MVIIA peptide was purified and freeze-dried, and the molecular weight was determined by Bruker BIFLEX MALDI-TOF-MS. The results showed that the pseudo ω-MVIIA peptide ([Sec 1,16 ] MVIIA, [Sec 8,20 ] MVIIA, [Sec 15,25 ]MVIIA) has a molecular weight of 2732.61Da, which is consistent with the theoretical molecular weight of 2733.02Da. It suggested that two of the six cysteines constituting ω-MVIIA were replaced by selenocysteine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com