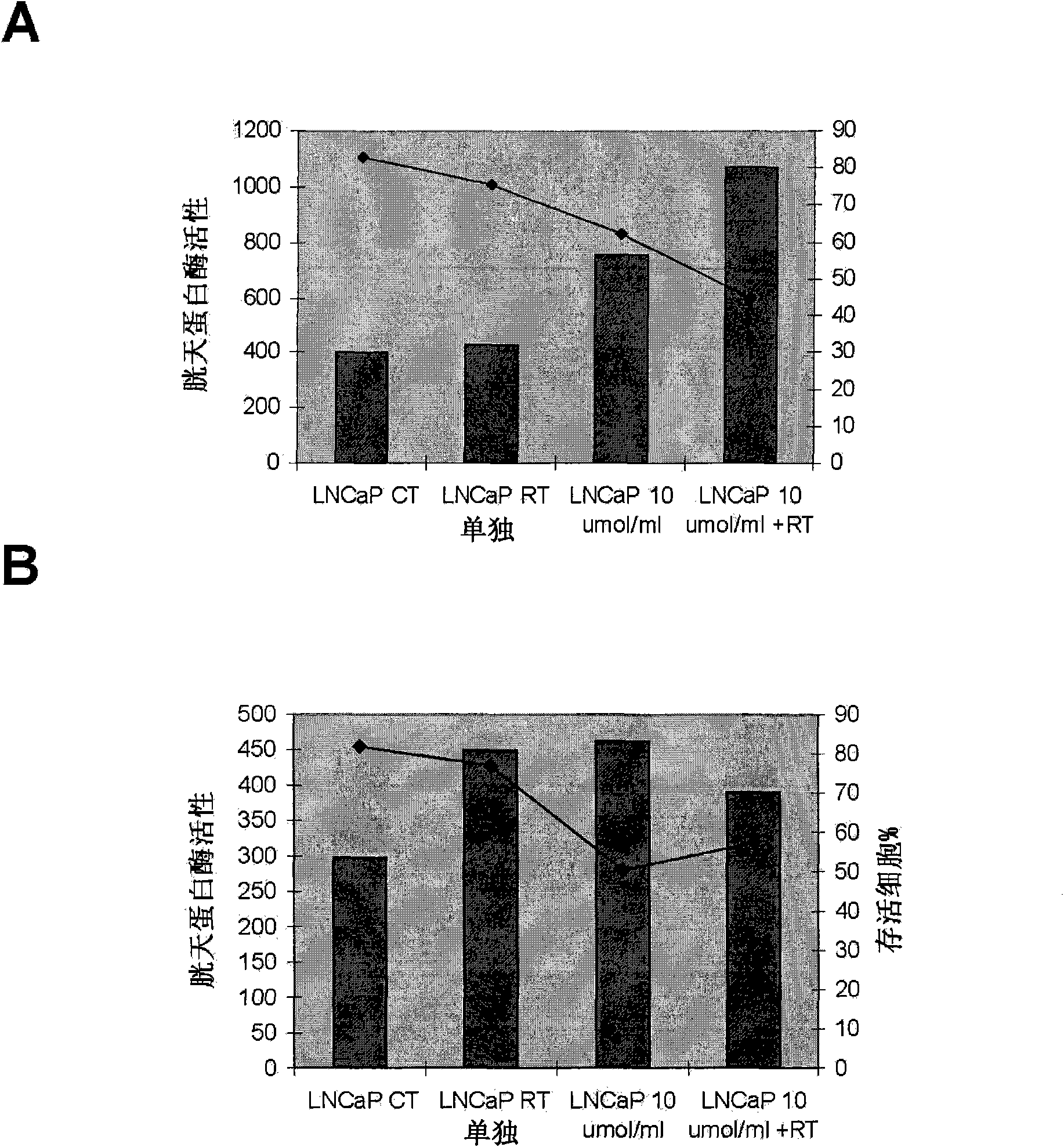
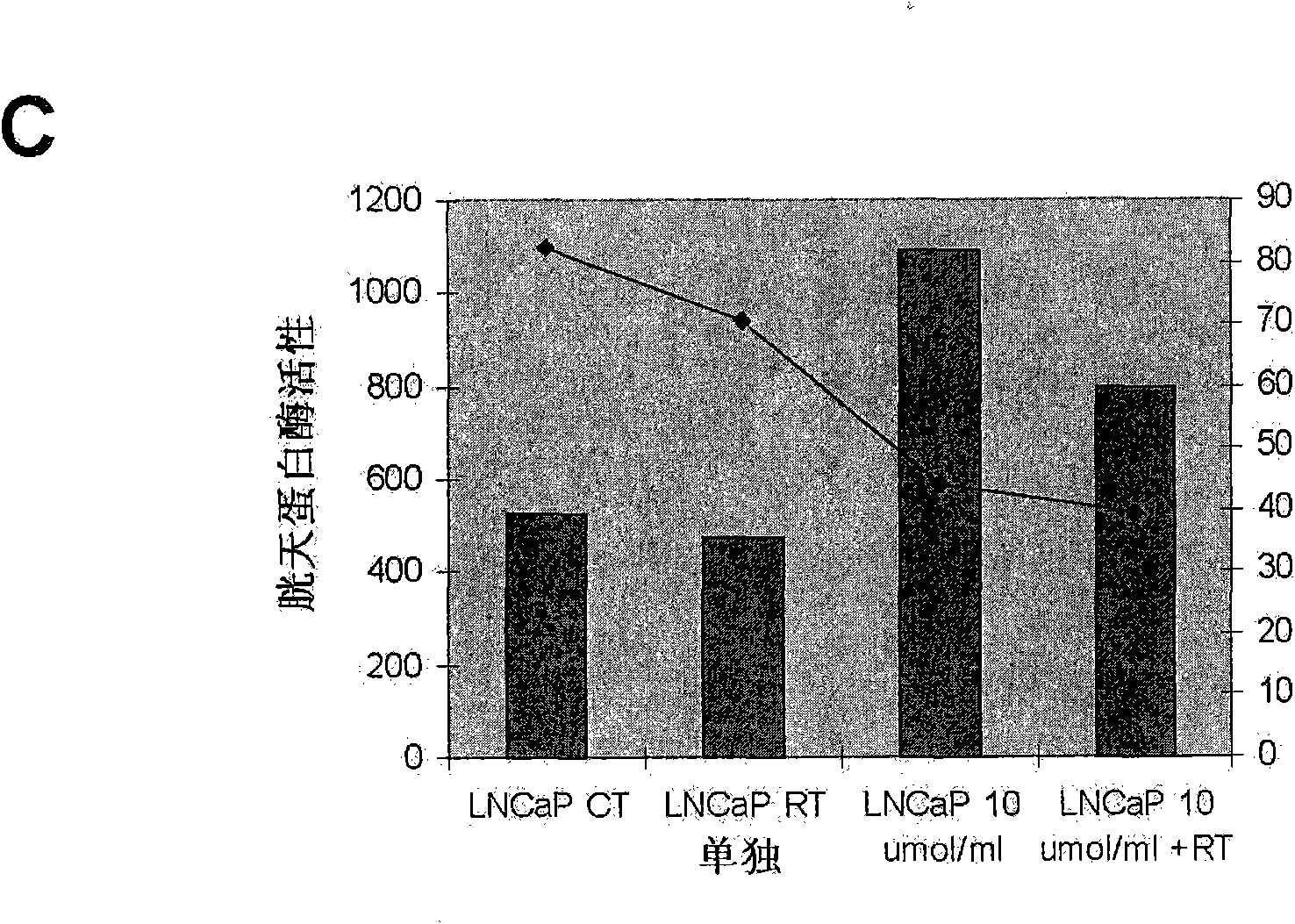
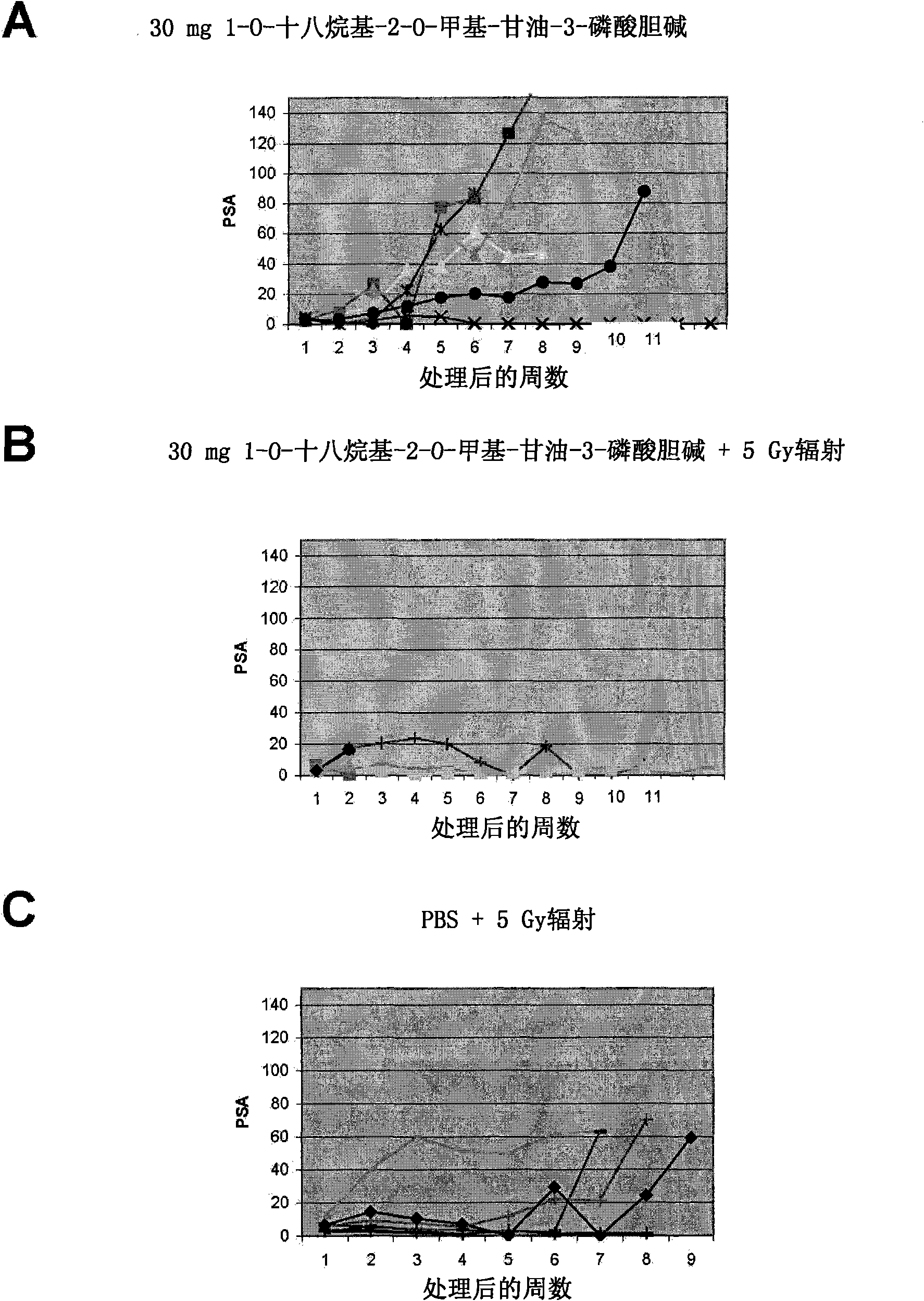
Methods and compositions for detecting receptor ligand mimetics
A technology for receptors and ligands, which is used in the field of detection of receptor ligand mimics and compositions, and can solve problems such as low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1: Screening steps
[0086] In order to verify the practical applicability of the method, as an example, we chose the FAS receptor to find its small activating ligand, the agonistic antibody CH-11 as a suitable receptor ligand, and AP-121 as the putative CH-11 Simulant. The known drugs camptothecin, temozolomide, doxorubicin and Tarceva were selected as a group of other compounds for combined measurement.
[0087] As the biological test group, we selected the brain tumor cell lines U87MG, A172, LN-18, LN-229, U118MG and T98G, which have different levels of mutations in p53 and PTEN and FAS receptor expression:
[0088] #
Cell line
p53
FasL
Fas
ATCC#
1
2
3
4
5
6
U87
A172
LN18
LN299
T98G
U-118MG
Mutant
Mutant
(Missing)
Mutant
(Missing)
Wild type
Mutant ...
Embodiment 2
[0095] Example 2: Cell lines and reagents
[0096] U-118MG (glioblastoma / astrocytoma, p53 mutation, PTEN mutation), T98G (glioblastoma multiforme, p53 mutation, PTEN mutation), A172 (glioblastoma, p53 wild Type, PTEN mutation) and U87MG (glioblastoma / astrocytoma, p53 wild type, PTEN mutation) were purchased from ATCC.
[0097] According to ATCC’s recommendation, U-118MG and A172 were incubated in DMEM medium supplemented with 10% FBS and P / S, and T98G and U87MG were supplemented with 0.1 mM non-essential amino acid solution (Gibco), 10% FBS and P / S. / S in MEM medium. AP-121 was prepared with sterile distilled water as a 5mM stock solution; Temozolomide (TMZ) (HaoruiPharma-Chem, Inc., Edison, NJ) was prepared with sterile filtered DMSO to make 100mM; Adriamycin (ADR) (Sigma) was used without Bacterial distilled water was prepared as 1 mM; camptothecin (CTP) (Sigma) was prepared as a 10 mM solution with 0.1N NaOH; Tarceva (protein kinase, Germany) was prepared as a 5 mM solution w...
Embodiment 3
[0098] Example 3: WST-1 proliferation analysis
[0099] 2000 cells were plated in each well of a 96-well flat bottom plate and incubated overnight at 37°C and 5% CO2. The growth of plated cells was determined by adding 7.5 μM WST-1 reagent (Roche Applied Sciences, Germany) to 3 control wells, and measuring the absorbance at 650 nm and 450 nm using a SpectraMax250 plate reader, respectively. If the OD650-OD450 value is higher than 0.5, the remaining wells of the plate can be used to incubate with AP-121, other agents or solvents for 96 hours.
[0100]After incubation, add WST-1 reagent to the well, and calculate the OD650-OD450 value as described above. Analyze 6 wells for each condition and determine the standard deviation: all experiments are performed independently at least three times. After elucidating the individual IC50 value of each compound, AP-121 and the chemotherapeutic agent were used for simultaneous combination treatment at their IC50 ratio; the combination index (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com