Method for preparing 2,4-dinitroaniline diazonium salt
A technology of dinitroaniline and diazonium salt, which is applied in the field of intermediate preparation of synthetic dyes, can solve problems such as decline, decomposition and explosion, dangerous product quality, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Diazotization: Take 135.3g of anhydrous sulfuric acid and slowly add dried NaNO at a temperature of 25°C 2 11.2g, reacted at 80°C for 2 hours. Cool down to below 40°C, then add 28g of 2,4-dinitroaniline with a content of 98% at 40°C, react for 6 hours, cool down to 10°C, dilute into ice-water mixture, control the temperature to ≤0°C, and obtain 2, 4-Dinitroaniline diazonium sulfuric acid aqueous solution.
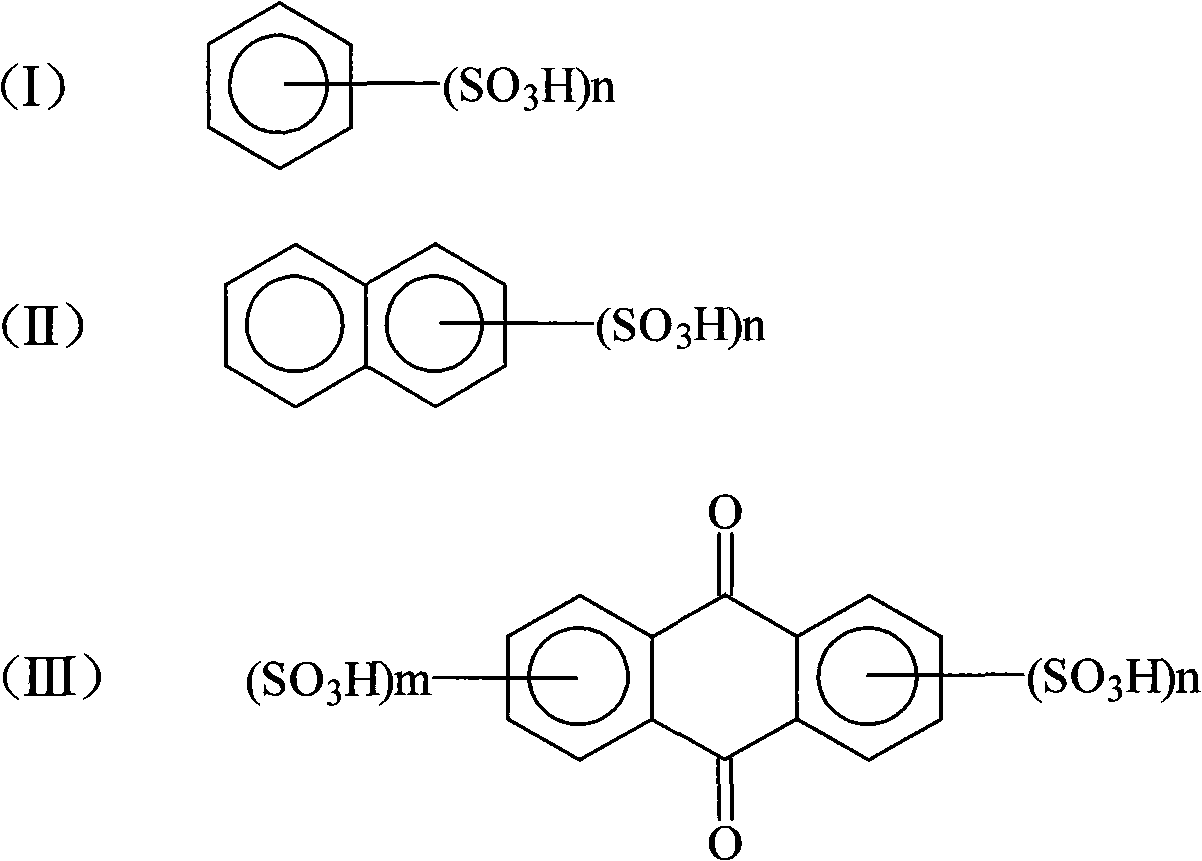
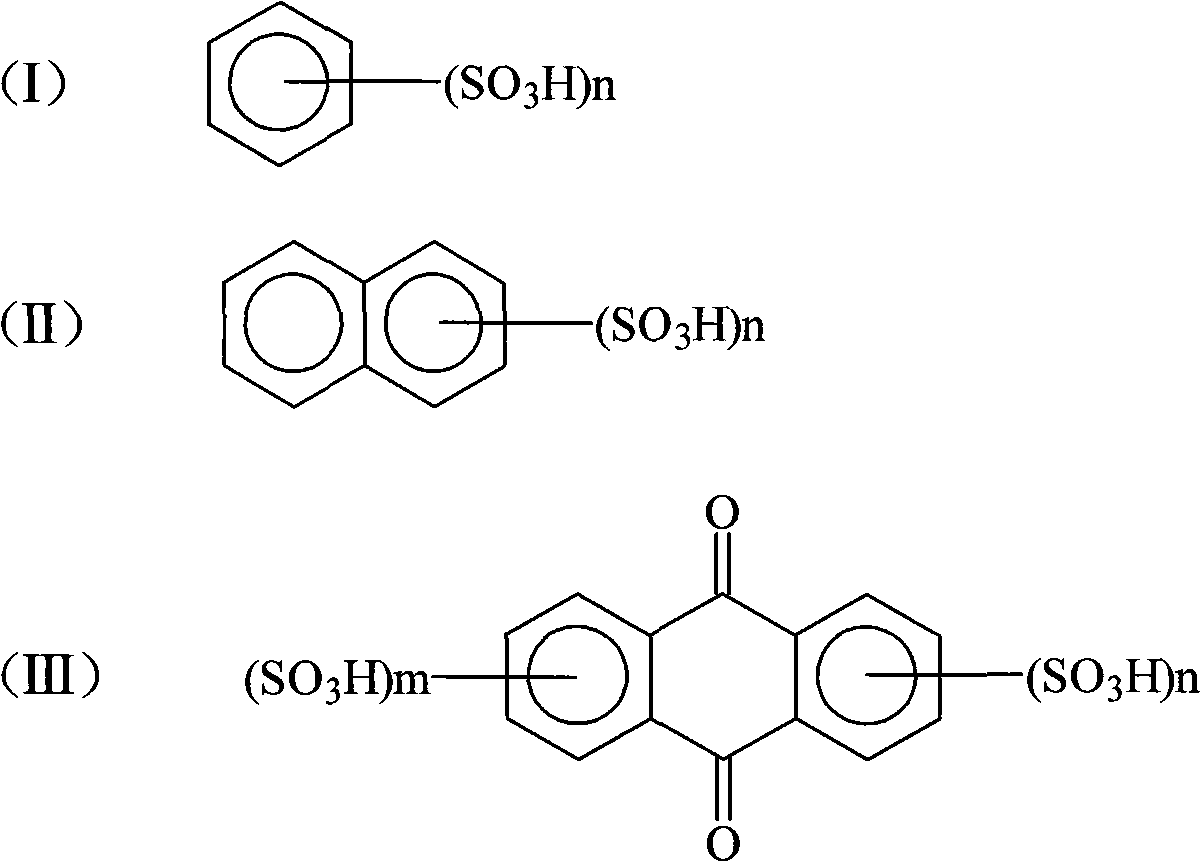
[0027] (2) Crystallization: In 2,4-dinitroaniline diazonium salt sulfuric acid aqueous solution, add 50g of benzenesulfonic acid, stir and precipitate for more than 2 hours, filter to get 2,4-dinitroaniline diazonium salt filter cake , to dry.
[0028] (3) Drying: Dry the filter cake of 2,4-dinitroaniline diazonium salt at about 80°C to obtain powdery 2,4-dinitroaniline diazonium salt, that is, the weight of 2,4-dinitroaniline Nitrogen salt solid powder.
Embodiment 2
[0030] (1) Diazotization: take 93.7g of 98% sulfuric acid, and mix it with 41.6g containing SO under cooling 3 20% oleum was mixed to make 135.3g of anhydrous sulfuric acid. In the anhydrous sulfuric acid, slowly add dried NaNO at a temperature ≤30°C 2 11.2g, reacted at 60°C for 3 hours. Cool down to below 40°C, then add 28g of 2,4-dinitroaniline with a content of 98% at 45°C, react for 7 hours, cool down to below 15°C, dilute into ice-water mixture, control the temperature to ≤0°C, and obtain 2 , 4-Dinitroaniline diazonium sulfuric acid aqueous solution.
[0031] (2) Crystallization: In 2,4-dinitroaniline diazonium salt sulfuric acid aqueous solution, add 30g of 1,5-naphthalene disulfonic acid and 20g sodium dodecylsulfonate, stir and precipitate for more than 2 hours, filter Afterwards, the filter cake of 2,4-dinitroaniline diazonium salt was obtained and dried.
[0032] (3) Drying: Dry the filter cake of 2,4-dinitroaniline diazonium salt at 85°C to obtain powdery 2,4-di...
Embodiment 3
[0034] (1) Diazotization: take 93.7g of 98% sulfuric acid, and mix it with 41.6g containing SO under cooling 3 20% oleum was mixed to make 135.3g of anhydrous sulfuric acid. In the anhydrous sulfuric acid, slowly add dried NaNO at a temperature ≤30°C 2 11.2g, reacted at 70°C for 2 hours. Cool down to below 40°C, then add 28g of 2,4-dinitroaniline with a content of 98% at 35°C, react 8, cool down to below 15°C, dilute into ice-water mixture, control the temperature to ≤0°C, and obtain 2, 4-Dinitroaniline diazonium sulfuric acid aqueous solution.
[0035] (2) Crystallization: Add 50 g of anthraquinone-2,5-disulfonic acid to 2,4-dinitroaniline diazonium sulfuric acid aqueous solution, stir and precipitate for more than 2 hours. Filter cake of 2,4-dinitroaniline diazonium salt and wait for drying.
[0036] (3) Drying: Dry the filter cake of 2,4-dinitroaniline diazonium salt below 75°C to obtain powdery 2,4-dinitroaniline diazonium salt, that is, the weight of 2,4-dinitroanilin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com