Fine silver particle, process for producing fine silver particle, and apparatus for producing fine silver particle
A manufacturing method and silver nanoparticle technology are applied in the fields of silver microparticles, the manufacture of silver microparticles, and the manufacture of silver microparticles, which can solve the problems of low manufacturing efficiency, narrowed flow path, increased loss, etc., and achieve efficient manufacturing, increase The effect of aggregating the number of center points and increasing the number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 Embodiment approach
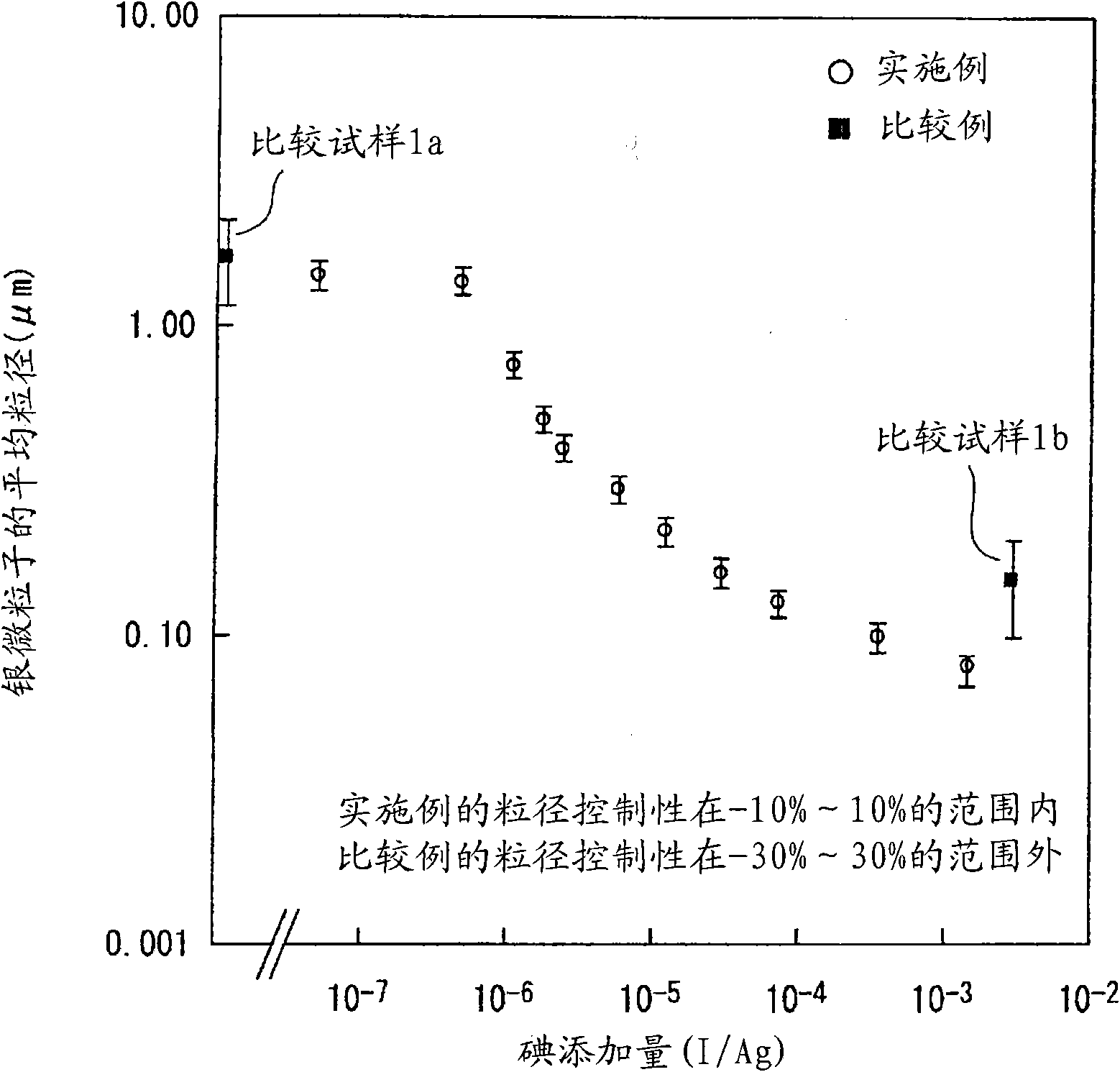
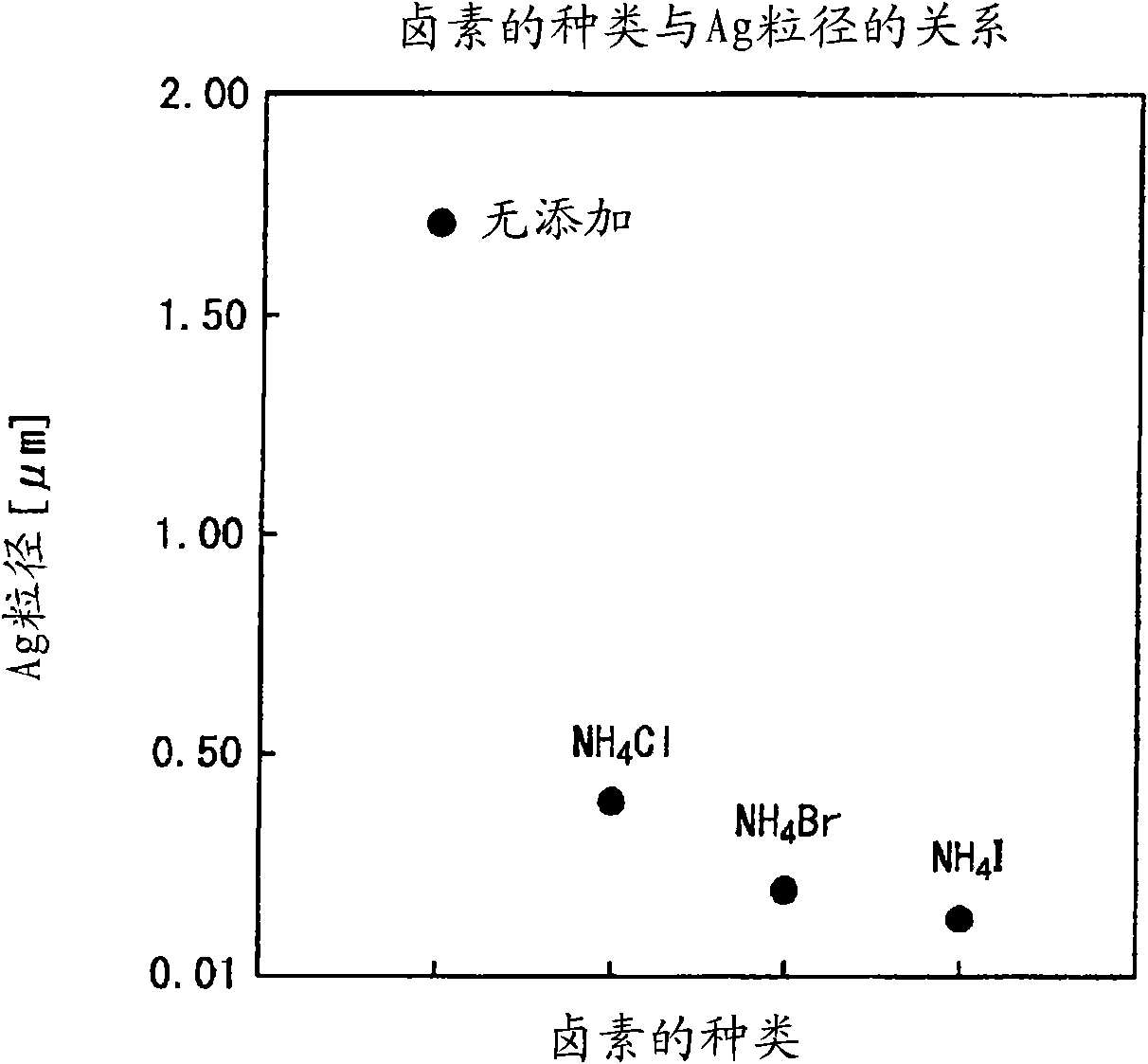
[0122] The silver microparticles of the present invention are silver microparticles produced by reducing silver ions in the presence of halide ions, and are 5.0×10 with respect to silver. -8 ~1.5×10 -3 The molar ratio contains halides, and it is a fine silver particle with good dispersibility.
[0123] 【0032】
[0124] In the method of adding a reducing agent to a silver ion solution to reduce silver ions to precipitate silver fine particles, the silver fine particles of the present invention can be produced by reducing silver ions in the presence of halide ions. In addition, in this production method, by adjusting the halide ion concentration with respect to the silver concentration, the particle size of the precipitated silver fine particles can be controlled.
[0125] 【0033】
[0126] As the silver ion solution, a silver nitrate solution added with ammonia water or the like can be used. Silver-ammonia complexes are formed due to the presence of ammonia, and silver is redu...
no. 2 Embodiment approach
[0151] The method for producing silver microparticles according to the second embodiment has a step of adding a reducing agent to a silver ion solution to reduce silver ions to precipitate silver microparticles, using a combination of a main reducing agent and a sub-reducing agent with a stronger reducing ability than the main reducing agent, and adding a reducing agent to the silver ion solution. In the ionic solution, the main reducing agent is added in the presence of a small amount of secondary reducing agent to precipitate tiny silver particles. By adjusting the addition amount of the secondary reducing agent, the particle size of the precipitated silver microparticles is controlled.
[0152] 【0045】
[0153] As the silver ion solution, a silver nitrate solution added with ammonia water or the like can be used. Silver-ammonia complexes are formed in this solution, and are reduced and precipitated by adding a reducing agent, silver.
[0154] 【0046】
[0155] In the method...
no. 3 Embodiment approach
[0174] The method for producing silver microparticles according to the third embodiment has a step of adding a reducing agent to a silver ion solution to reduce silver ions to precipitate silver microparticles, and by adding silver nanoparticles and reducing silver ions in the presence of the silver nanoparticles, tiny silver particles Microparticles precipitated. By adjusting the amount of silver nanoparticles added to the silver concentration, the particle size of the precipitated silver microparticles can be controlled.
[0175] 【0055】
[0176] As the silver ion solution, a silver nitrate solution added with ammonia water or the like can be used. Silver-ammonia complexes are formed in this solution, and are reduced and precipitated by adding a reducing agent, silver. As the reducing solution, a solution of an organic reducing agent having a phenolic hydroxyl group such as a hydroquinone solution, a pyrogallol solution, or a 3,4-dihydroxyphenol solution can be used.
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com