Method for synthesizing 2, 5-dichloro-phthaldialdehyde
A technology of dichloroterephthalaldehyde and dichloro-p-xylene, applied in two fields, can solve problems such as low yield, unreported synthesis method, corrosion pollution, etc., achieve short reaction route, low cost, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0020] The present invention will be further described below in conjunction with specific examples, but the present invention is not limited to these specific examples.
example 1
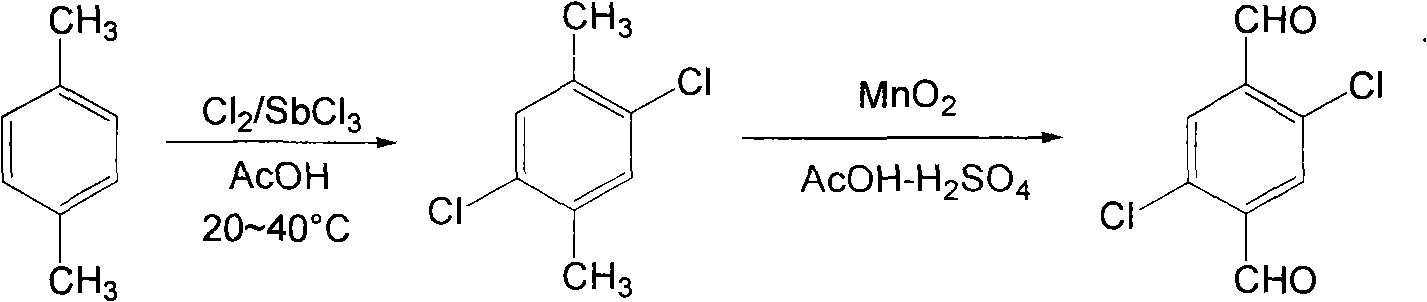
[0022] Preparation of 2,5-dichloro-p-xylene: Add 200g of acetic acid, 2.3g of antimony trichloride and 21.3g of p-xylene into a 500ml round bottom flask. Heat and stir, control the temperature at 20-40°C, and control the chlorine flow rate by bubbling. Keep the reaction below 40°C, continue to pass chlorine for 2 to 4 hours, and the reaction solution is yellow-green. Recrystallization gave 34.1 g of 2,5-dichloro-p-xylene (melting point: 69-70°C, yield: 97.5%).
[0023] The preparation of 2,5-dichloroterephthalaldehyde: add 35g 2,5-dichloro-p-xylene in 220ml acetic acid, heat and stir until fully dissolved, then add 300ml70% sulfuric acid solution, add 80g manganese dioxide in batches, Continue to raise the temperature to reflux, react for 8 hours, filter out the insoluble matter, extract, wash, dry, and distill under reduced pressure to collect 34.8g of the fraction product at 183-187°C under 0.005MPa, which is 2,5-dichloro-terephthalene Formaldehyde (melting point 157-158°C...
example 2
[0025] Preparation of 2,5-dichloro-p-xylene: Add 200g of acetic acid, 2.6g of antimony trichloride and 21.3g of p-xylene into a 500ml round bottom flask. Heat and stir, control the temperature at 20-40°C, and control the chlorine flow rate by bubbling. Keep the reaction at no more than 40°C, continue to pass chlorine for 5 hours, and the reaction solution is yellow-green. Recrystallization gave 34.4 g of 2,5-dichloro-p-xylene (melting point: 69-70°C, yield: 98.1%).
[0026] Preparation of 2,5-dichloroterephthalaldehyde: add 35g 2,5-dichloro-p-xylene to 100ml acetic acid, heat and stir until completely dissolved, then add 250ml 70% sulfuric acid solution, add 74g manganese dioxide in batches , continue to heat up to reflux, react for 5 to 7 hours, filter out insoluble matter, extract, wash, dry, and distill under reduced pressure to collect 35.2 g of the fraction product at 183 to 187 °C under 0.005 MPa, which is 2,5-dichloro Terephthalaldehyde (melting point 157-158°C, yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com