Production method of fodder calcium phosphate salt
The technology of a calcium phosphate salt and a production method is applied in the production field of feed calcium phosphate salt, which can solve the problems of incomplete reaction, single product type and high cost, and achieve easy control of the production process, wide process adaptability and low production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
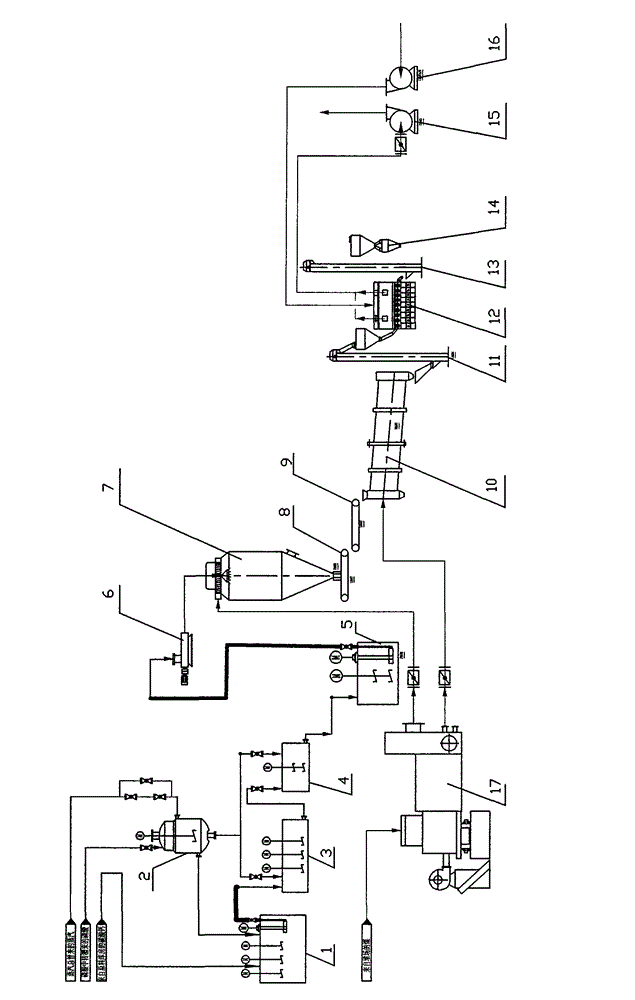
[0017] Take the annual output of 50,000 tons of calcium dihydrogen phosphate as an example:
[0018] Limestone (CaCO 3 Content is 98%) 200 mesh powder enters re-sizing tank 1, and adds water in re-sizing tank 1, controls the slurry solid content of re-sizing tank 1 to be about 65%, is heated to make the slurry temperature be 105 ℃, then use The pump transports the slurry to the continuous reaction tank 3 with a stirring device at a flow rate of 4.708 tons per hour; the purified phosphoric acid with a concentration of 32% first enters the acid heater 2 to raise the temperature to 105 ° C, and the flow rate is 10.994 tons per hour. The flow enters the continuous reaction tank 3 to react with the slurry, and the reaction time is 60 minutes; the reacted slurry enters the slurry tank 4, and then adds the above-mentioned purified phosphoric acid to the slurry tank 4 for further reaction at a flow rate of 7.328 tons per hour , the reaction temperature is controlled at 105 DEG C, and...
Embodiment 2
[0020] Take the annual output of 30,000 tons of calcium dihydrogen phosphate as an example:
[0021] Limestone (CaCO3 content is 99%) 400-mesh powder from the raw material warehouse enters the re-sizing tank 1, and water is added in the re-sizing tank 1, and the solid content of the slurry in the re-sizing tank 1 is controlled to be about 70%, and heating makes the slurry The temperature is 90°C, and then pump the slurry to the continuous reaction tank 3 with a stirring device at a flow rate of 2.596 tons per hour; the purified phosphoric acid with a concentration of 54% first enters the acid heater 2 to raise the temperature to 90°C , enter the continuous reaction tank 3 with a flow rate of 3.909 tons per hour to react with the slurry, and the reaction time is 20 minutes; the reacted slurry enters the slurry tank 4, and goes to the slurry tank 4 with a flow rate of 2.606 tons per hour Then add the above-mentioned purified phosphoric acid for further reaction, the reaction tem...
Embodiment 3
[0023] Take the annual output of 30,000 tons of calcium hydrogen phosphate as an example:
[0024] Limestone (CaCO3 content is 98.5%) 350-mesh powder from the raw material warehouse enters the re-sizing tank 1, and water is added to the re-sizing tank 1, and the solid content of the slurry in the re-sizing tank 1 is controlled to be about 68%, and heating makes the slurry The temperature is 100°C, and then pump the slurry to the continuous reaction tank 3 with a stirring device at a flow rate of 4.576 tons per hour; the purified phosphoric acid with a concentration of 46% first enters the acid heater 2 to raise the temperature to 100°C , enter the continuous reaction tank 3 with a flow rate of 3.917 tons per hour to react with the slurry, and the reaction time is 40 minutes; the reacted slurry enters the slurry tank 4, and goes to the slurry tank 4 with a flow rate of 2.611 tons per hour Add the above-mentioned purified phosphoric acid for further reaction, the reaction temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com