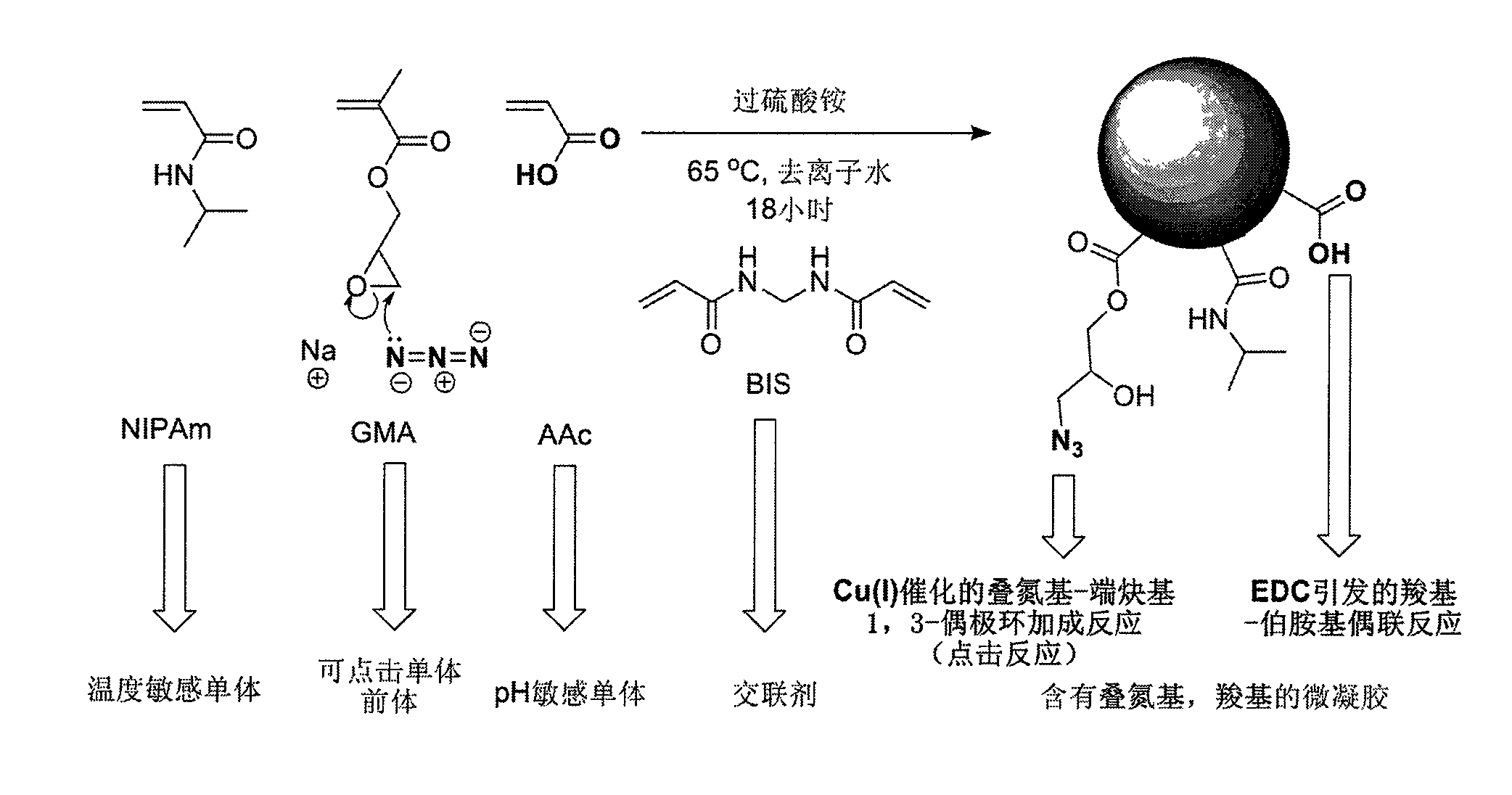
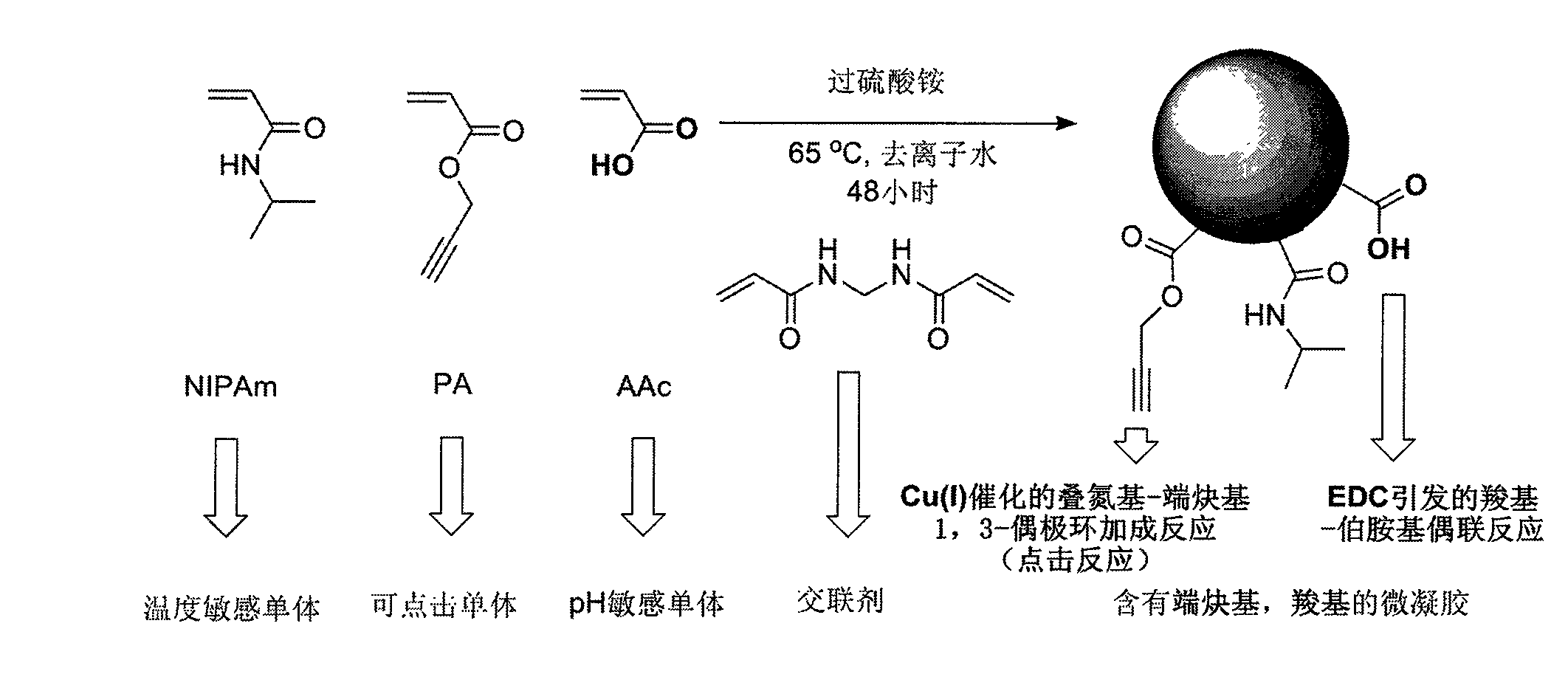
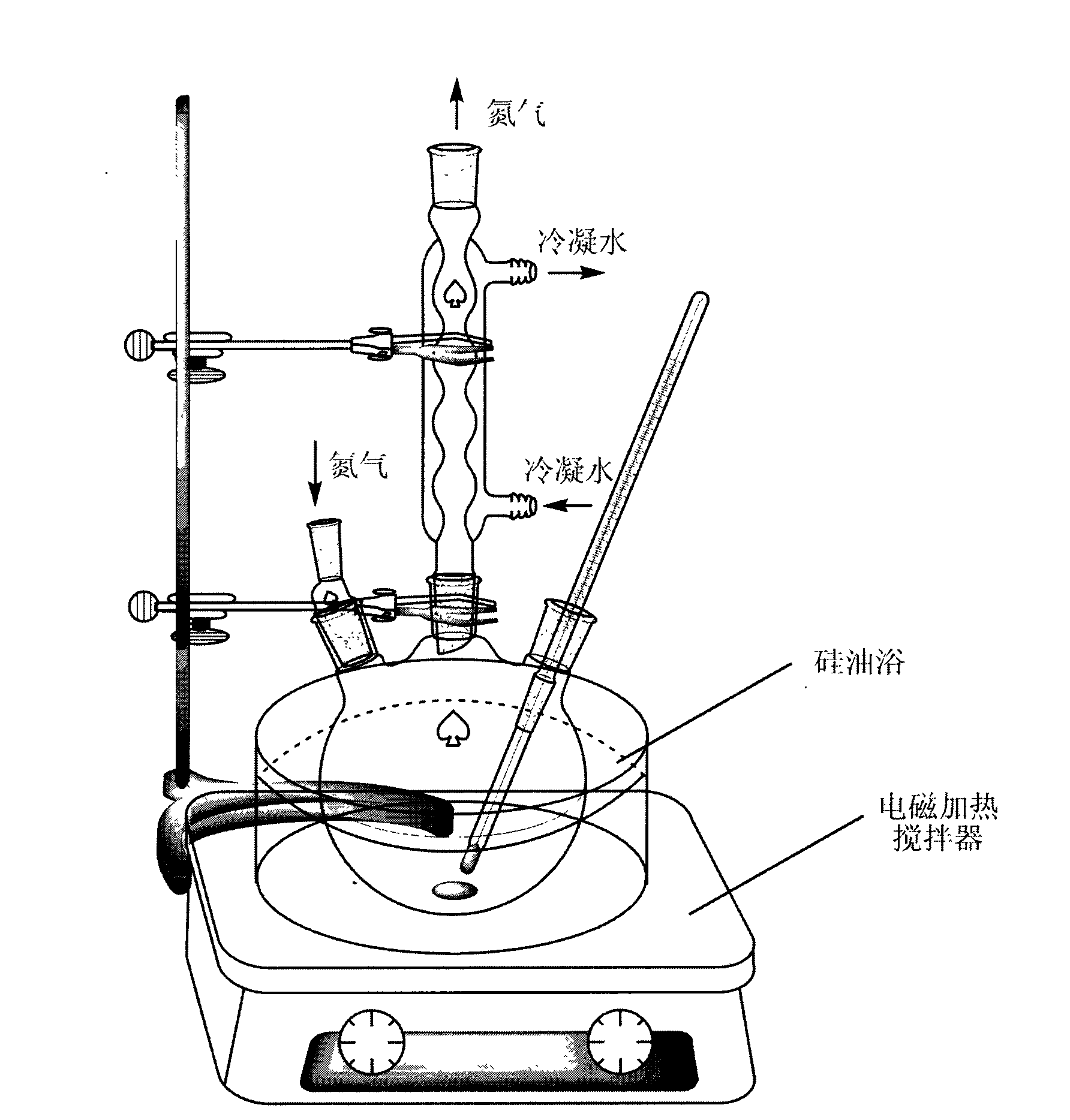
Point-and-click temperature/pH-sensibility microgel
A Microgel, Sensitive Technology for Applications in Polymer Synthetic Chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034]First, weigh out 1.6 g of N-isopropylacrylamide (NIPAm), 200 microliters of acrylic acid (AAc), and 0.04 g of N,N-methylenebisacrylamide (BIS) with an analytical balance, and use 60 ml of deionized water Dissolve in a 200ml beaker, and if necessary, ultrasonically vibrate for 30 seconds to speed up the dissolution process. The above solution was then transferred into a 250 mL three necked round bottom flask via a syringe with a 0.8 micron pore size filter. The beaker was rinsed with an additional 30 mL of deionized water and sonicated, and the rinsed solution was transferred into a 250 mL three-neck round bottom flask through a 0.8 micron filter syringe. At this time, the flask was set in a silicone oil bath, nitrogen gas was introduced into the left bottle mouth, a thermometer was inserted into the right bottle mouth, and a condenser tube with a nitrogen outlet was connected to the middle bottle mouth, which was fixed on an iron stand. The temperature of the silicone o...
example 2
[0036] First, 1.6 grams of N-isopropylacrylamide (NIPAm), 200 microliters of acrylic acid (AAc), and 0.04 grams of N,N'-methylenebisacrylamide (BIS) were weighed out with an analytical balance, and 60 milliliters of deionized Dissolve water in a 200ml beaker, and if necessary, use a sonicator to sonicate for 30 seconds to speed up the dissolution process. The above solution was then transferred into a 250 mL three necked round bottom flask via a syringe with a 0.8 micron pore size filter. The beaker was rinsed with an additional 30 mL of deionized water and sonicated, and the rinsed solution was transferred into a 250 mL three-neck round bottom flask through a 0.8 micron filter syringe. At this time, the flask was set in a silicone oil bath, nitrogen gas was introduced into the left bottle mouth, a thermometer was inserted into the right bottle mouth, and a condenser tube with a nitrogen outlet was connected to the middle bottle mouth, which was fixed on an iron stand. The te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com