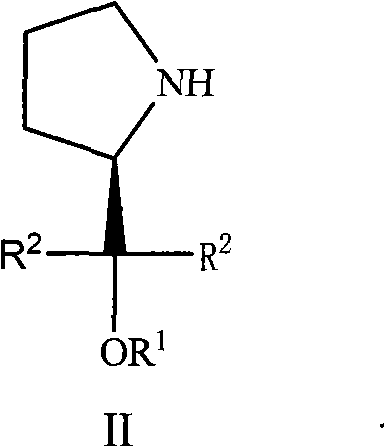
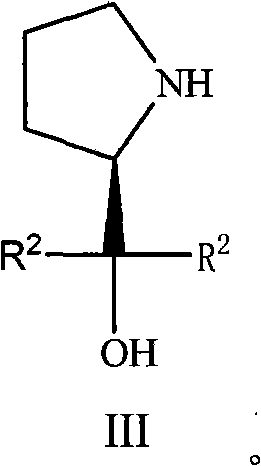
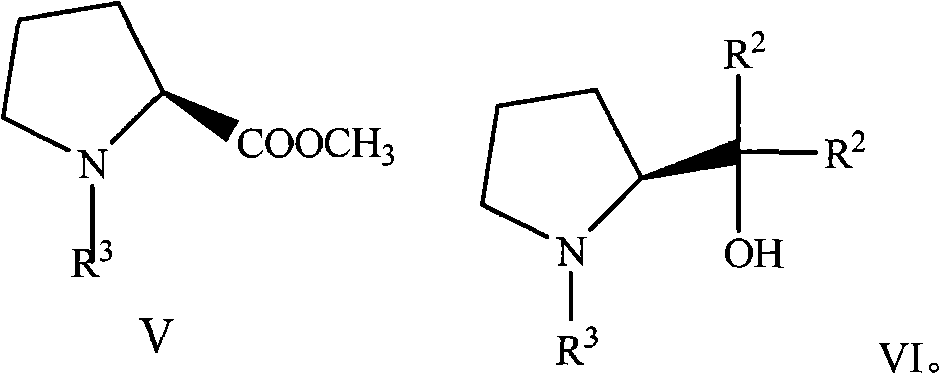
(S)-Alpha, Alpha-fluorine-containing diaryl-2-pyrrolidine methanol derivative as well as preparation and applications thereof
A technology for pyrrolidine methanol and derivatives, which is applied in the preparation of organic compounds, the preparation of carboxylic acid esters, and the activation/preparation of catalysts, can solve the problems of expensive catalysts, difficult recovery of catalysts, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of Optically Pure (S)-α,α-bis(3,4,5-fluorobenzene)-trimethylsilyl ether 2-pyrrolidine catalyst
[0053] 1. Synthesis of L-proline methyl ester (b)
[0054]
[0055] Add 40mmol (46g) of L-proline (a) and 30mL of methanol to the reaction flask, cool in an ice bath, slowly add 100mmol (118g) of thionyl chloride dropwise while stirring, after dropping, heat and reflux for 6 hours, reduce The solvent was distilled off under pressure to obtain a light yellow oily liquid, which was the crude product (b) of L-proline methyl ester, which was directly used in the next reaction.
[0056] 2. Synthesis of (S)-N-benzyl-2-pyrrolidinecarboxylic acid methyl ester (c)
[0057]
[0058] The crude product (b) from the previous step was dissolved in 40 mL of methanol, 78 mmol (82.6 g) of sodium carbonate was added, 46 mmol (58 g) of benzyl chloride was added dropwise under reflux, and reflux was continued for 4 hours after the dropwise addition. After the reaction was comp...
Embodiment 2
[0072] Other operations are the same as in Example 1, except that in step 4, the protic solvent is changed to isopropanol, and the reaction conditions are as follows.
[0073] Preparation of (S)-α,α-(3,4,5-trifluoro)diphenyl-2-pyrrolidinemethanol (e)
[0074] Take 10 mmol of compound (d) from the previous step, add 0.45 g of 10 wt% palladium carbon, 20 mL of isopropanol, and stir at room temperature under the protection of hydrogen. After reacting for 2 hours, the palladium carbon was removed by filtration, and the solvent was evaporated under reduced pressure to obtain the pure product (S)-α, α-(3,4,5-trifluoro)diphenyl-2-pyrrolidinemethanol (e) 9.3 mmol, the yield is 93%.
Embodiment 3
[0076] Other operations are the same as in Example 1, except that in step 4, the protic solvent is changed to ethanol, and the reaction conditions are as follows.
[0077] (S)--α, α-(3,4,5-trifluoro)diphenyl-2-pyrrolidinemethanol (e) preparation
[0078] Take 10 mmol of compound (d) in the previous step, add 0.45 g of 10 wt% palladium carbon, 40 mL of ethanol, and stir at room temperature under the protection of hydrogen. After reacting for 4 hours, the palladium carbon was removed by filtration, and the solvent was evaporated under reduced pressure to obtain the pure product (S)-α, α-(3,4,5-trifluoro)diphenyl-2-pyrrolidinemethanol (e) 9.6 The mmol yield was 96%.
[0079] Subsequent operations and reaction conditions were the same as in Example 1 to obtain (S)-α,α-bis(3,4,5-fluorobenzene)-trimethylsilyl ether-2-pyrrolidine with a total yield of 38.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com