Method for production of quaternary ammonium hydroxide
A technology of quaternary ammonium base and halogenated quaternary ammonium salt, which is applied in chemical instruments and methods, electrolytic organic production, electrolytic components, etc., and can solve problems such as difficulty in completely isolating halogen gas and oxidation degradation of anion exchange membranes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] According to the following recipe, a paste mixture (polymerizable composition) for anion exchange membrane formation was prepared.
[0131] Recipe for the paste mixture:
[0132] 50 parts by weight of chloromethyl styrene
[0133] 50 parts by weight of styrene
[0134] 21.4 parts by weight of divinylbenzene
[0135] Di-tert-butyl peroxide (radical polymerization initiator) 7.14 parts by weight
[0136] Styrene-butadiene copolymer (base resin) 14.3 parts by weight
[0137] Next, a calendered high-density polyethylene net of the following specifications was prepared as a skeleton base material.
[0138] High-density polyethylene mesh:
[0139] Product name: NBC Industries ニツプ strong net
[0140] Thickness: 150μm
[0141] Mesh number: 200 per 1 square meter
[0142] The previously prepared paste mixture was coated on the above-mentioned skeleton substrate, followed by covering with a polyester film (peeling film), and then the paste mixture was polymerized to obt...
Embodiment 2
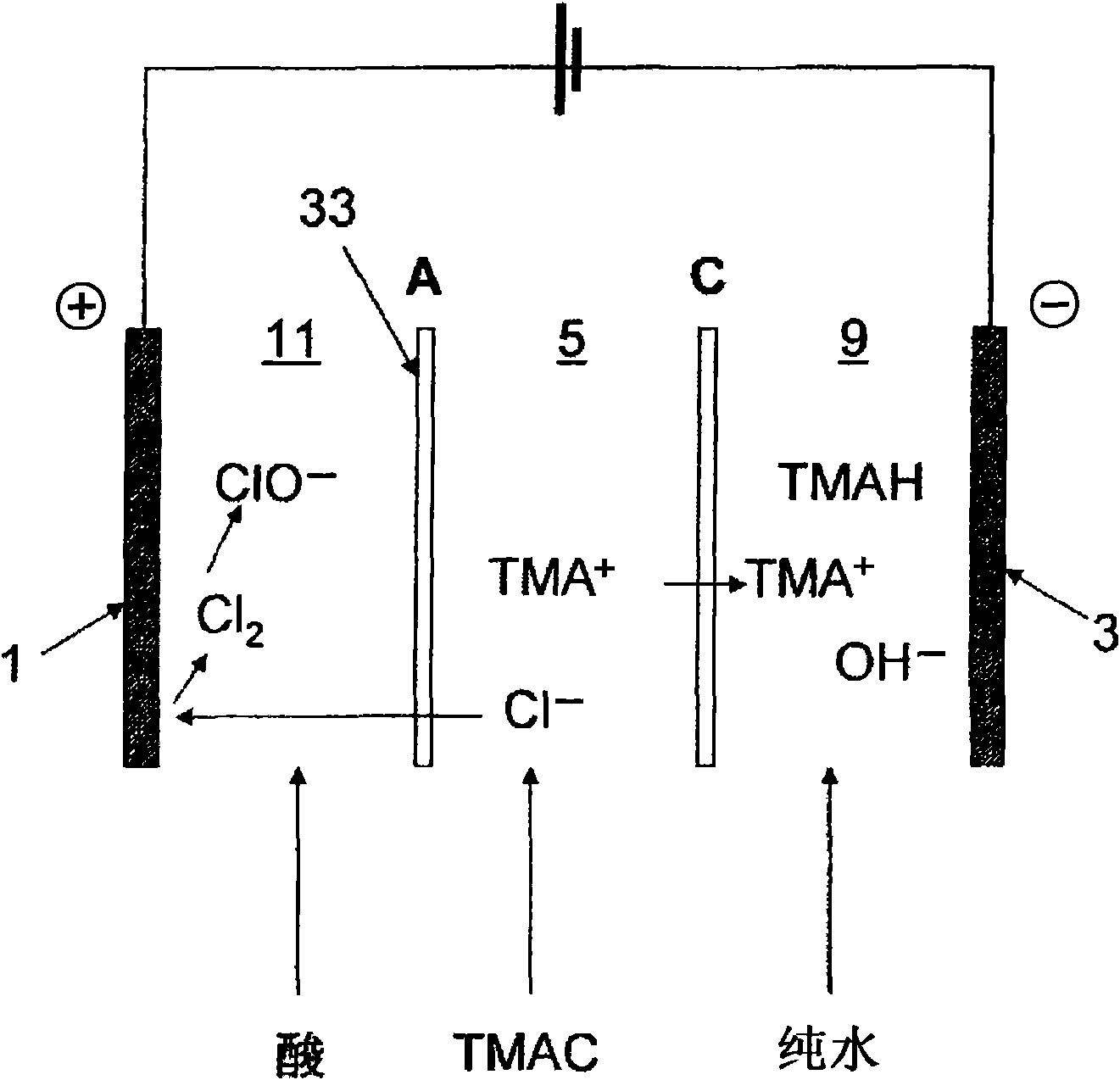
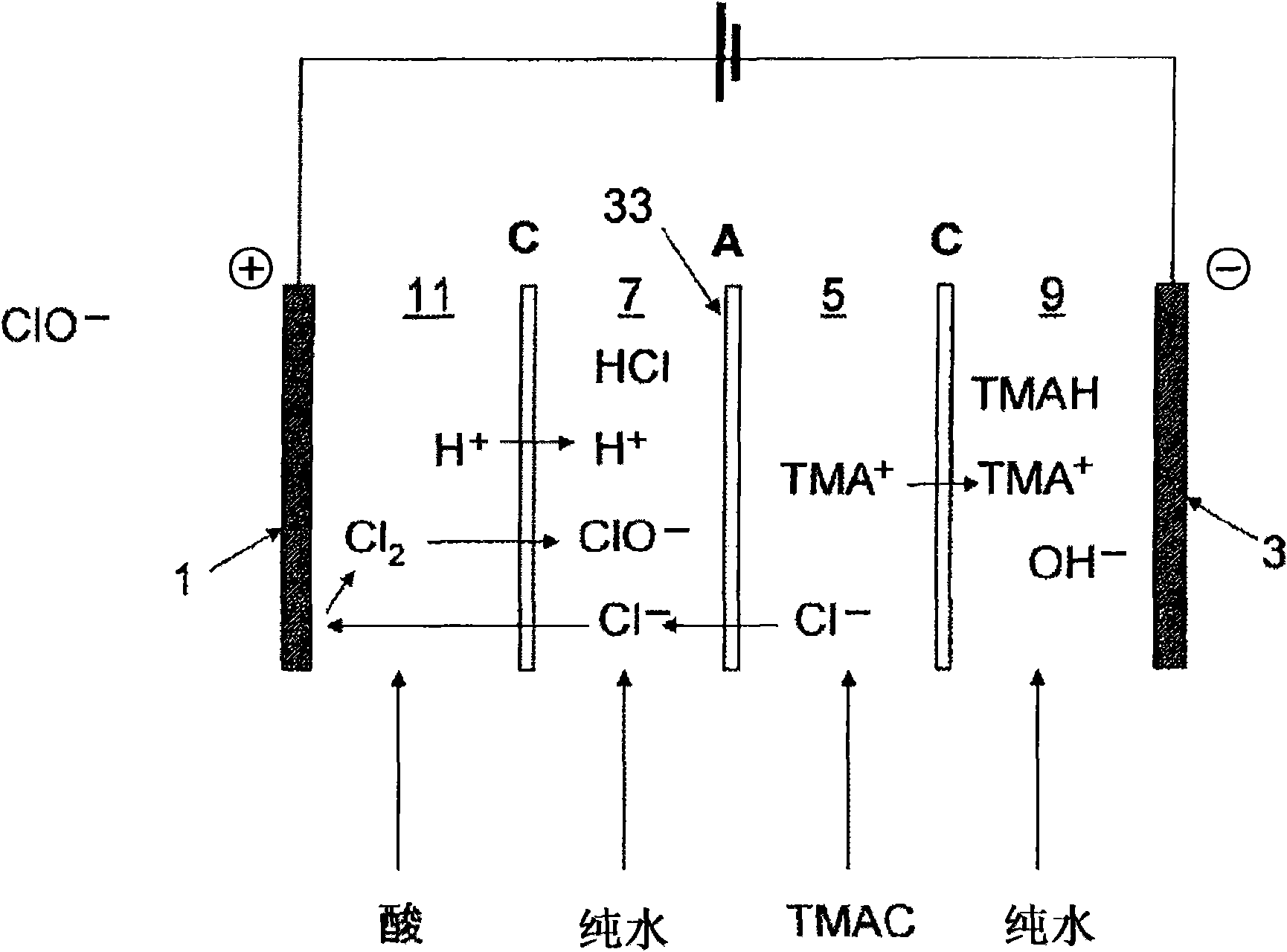
[0153]The anion exchange membrane A and cation exchange membrane C used in Example 1 were used except that figure 2 The setup shown has an effective membrane area of 2dm 2 The electrolysis was performed in the same manner as in Example 1 except in the electrolysis device. (The anion exchange membrane A is set so that the surface cross-linked layer is on the anode side.)
[0154] That is, in figure 2 0.5 equivalent of sulfuric acid circulates in the anode chamber 11 of the shown electrolysis device, circulates 0.5 equivalent of hydrochloric acid in the chamber 7 between the cation exchange membrane C and the anion exchange membrane A on the anode side, and circulates between the anion exchange membrane A and the cathode side Circulate 2.5 equivalents of tetramethylammonium chloride aqueous solution in the chamber (stock solution chamber 5) between the cation exchange membranes C, and circulate pure water in the cathode chamber 9, the current density is 30A / dm 2 , the tem...
Embodiment 3
[0157] An anion exchange membrane A was prepared in the same manner as in Example 1, except that the surface crosslinked layer was formed on one side of the original membrane by immersion in a 1.0-N dimethylamine aqueous solution for 3 hours.
[0158] The exchange capacity of the surface crosslinked layer at this time was 0.008 meq / g dry film. In addition, the ion-exchange capacity of the anion-exchange membrane A before electrolysis is 1.2meq / g dry membrane, the rupture strength of Mullen's formula is more than 1.0MPa, and the water permeability is 0ml / (m 2 ·hour·0.1MPa).
[0159] Except for using the above-mentioned anion exchange membrane A, the electrolytic operation was carried out continuously in exactly the same manner as in Example 2. As a result, after one year of continuous operation of the anion exchange membrane A, the ion exchange capacity was 1.0 meq / g dry membrane, Mullen The bursting strength of the formula is 0.9MPa, and the water permeability is 0ml / (m 2 ·h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Bursting strength | aaaaa | aaaaa |
| Bursting strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com