Methylphenidatefrozen dry powder preparation for injection and preparation process thereof
A technology of freeze-dried powder injection and dexmethylphenidate, which is applied in the field of dextromethylphenidate freeze-dried powder injection and its preparation, and can solve problems such as inability to achieve therapeutic effect, unstable medicine, and slow onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
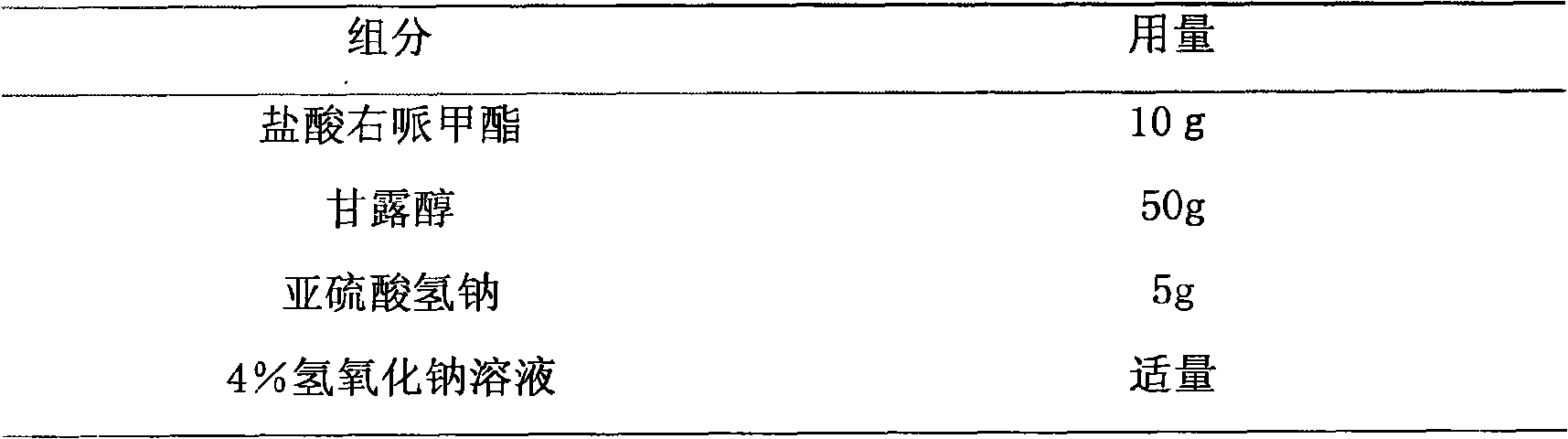
[0013] Embodiment 1 Dexmethylphenidate hydrochloride freeze-dried powder injection
[0014] prescription:
[0015]
[0016]
[0017] Preparation:
[0018] Take by weighing the prescribed amount of sodium bisulfite, dexmethylphenidate hydrochloride and add 80% water for injection to dissolve to make a solution of dexmethylphenidate hydrochloride, add the prescribed amount of mannitol, adjust the pH value to 3.0-9.5, and heat the medicinal solution to At about 60°C, add 0.1% activated carbon for needles according to the prepared amount, stir for 30 minutes, filter and decarbonize, and then use a 0.22 μm microporous membrane for fine filtration. After the intermediate is qualified, it is aseptically filled in a 10ml vial (per The theoretical volume of the bottle is 5ml), put the liquid in a freeze-drying box, and freeze for 4 hours, so that the temperature drops to about -45°C; the first sublimation takes 12 hours, and the temperature rises to about -5°C; the second sublim...
Embodiment 2
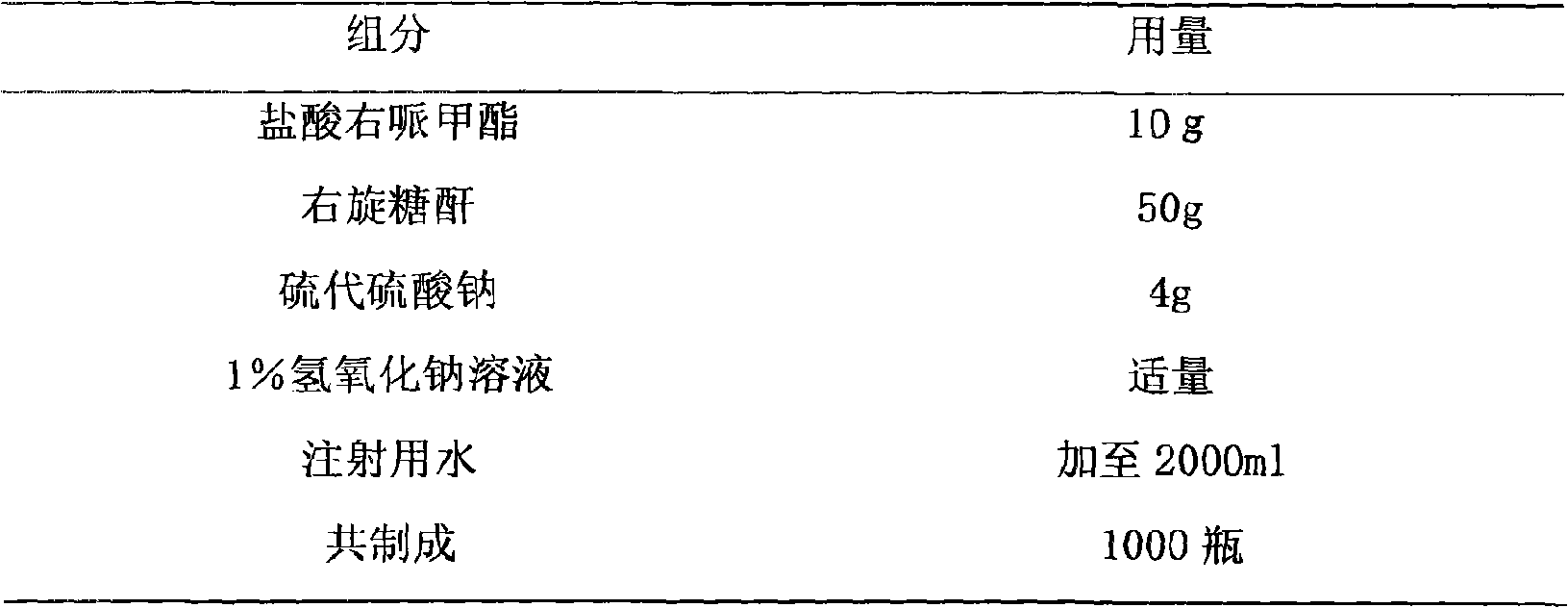
[0019] Embodiment 2: Dexmethylphenidate hydrochloride freeze-dried powder injection
[0020] prescription:
[0021]
[0022] Preparation:
[0023] Weigh the prescribed amount of sodium thiosulfate and dexmethylphenidate hydrochloride and add it to 80% water for injection heated to about 60°C to dissolve, then add the prescribed amount of dextran, adjust the pH value to 3.0-9.5, add 0.1% injection according to the prepared amount Activated carbon, insulated and stirred for 30 minutes, filtered, decarbonized, and then finely filtered with a 0.22 μm microporous membrane. After the intermediate was tested and qualified, it was aseptically filled in a 5ml vial (theoretical amount of each bottle was 2ml), and the liquid was placed in Freeze in a freeze-drying box for 3 hours to lower the temperature to about -45°C; for the first sublimation for 8 hours, the temperature rises to about -5°C; for the second sublimation for 4 hours, the temperature rises to 30°C, after vacuum cappin...
Embodiment 3
[0024] Embodiment 3: Dexmethylphenidate hydrochloride freeze-dried powder injection
[0025] prescription:
[0026]
[0027]
[0028] Preparation:
[0029] Weigh the prescribed amount of dexmethylphenidate hydrochloride and add it to 80% water for injection heated to about 60°C to dissolve, then add the prescribed amount of glycine, sodium metabisulfite, sodium calcium edetate, stir to dissolve and adjust the pH value to 3.0-9.5 , add 0.1% activated carbon for needles according to the prepared amount, heat and stir for 30 minutes, filter, decarbonize, and then finely filter with a 0.22 μm microporous membrane. After the intermediate is qualified, it is aseptically filled in a 5ml vial (each bottle Theoretical amount is 2ml), put the drug solution in a freeze-drying box, and freeze for 3 hours, so that the temperature drops to about -45°C; the first sublimation takes 14 to 16 hours, and the temperature rises to about -5°C; the second sublimation is 4~ After 6 hours, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com