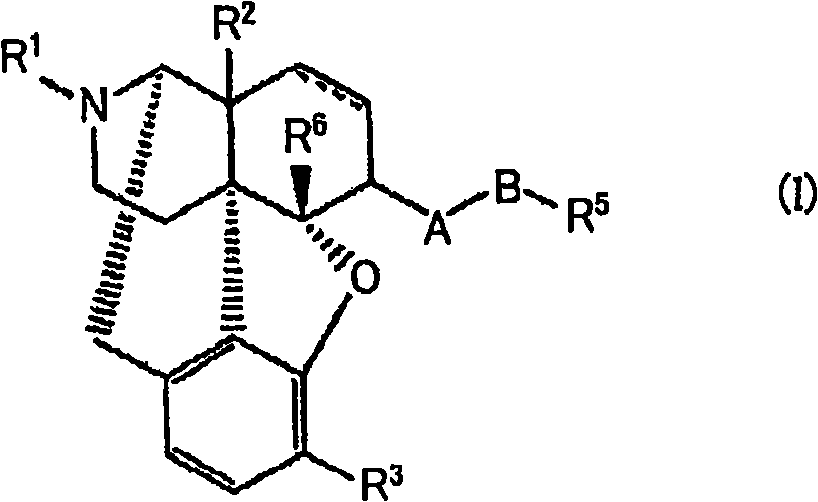
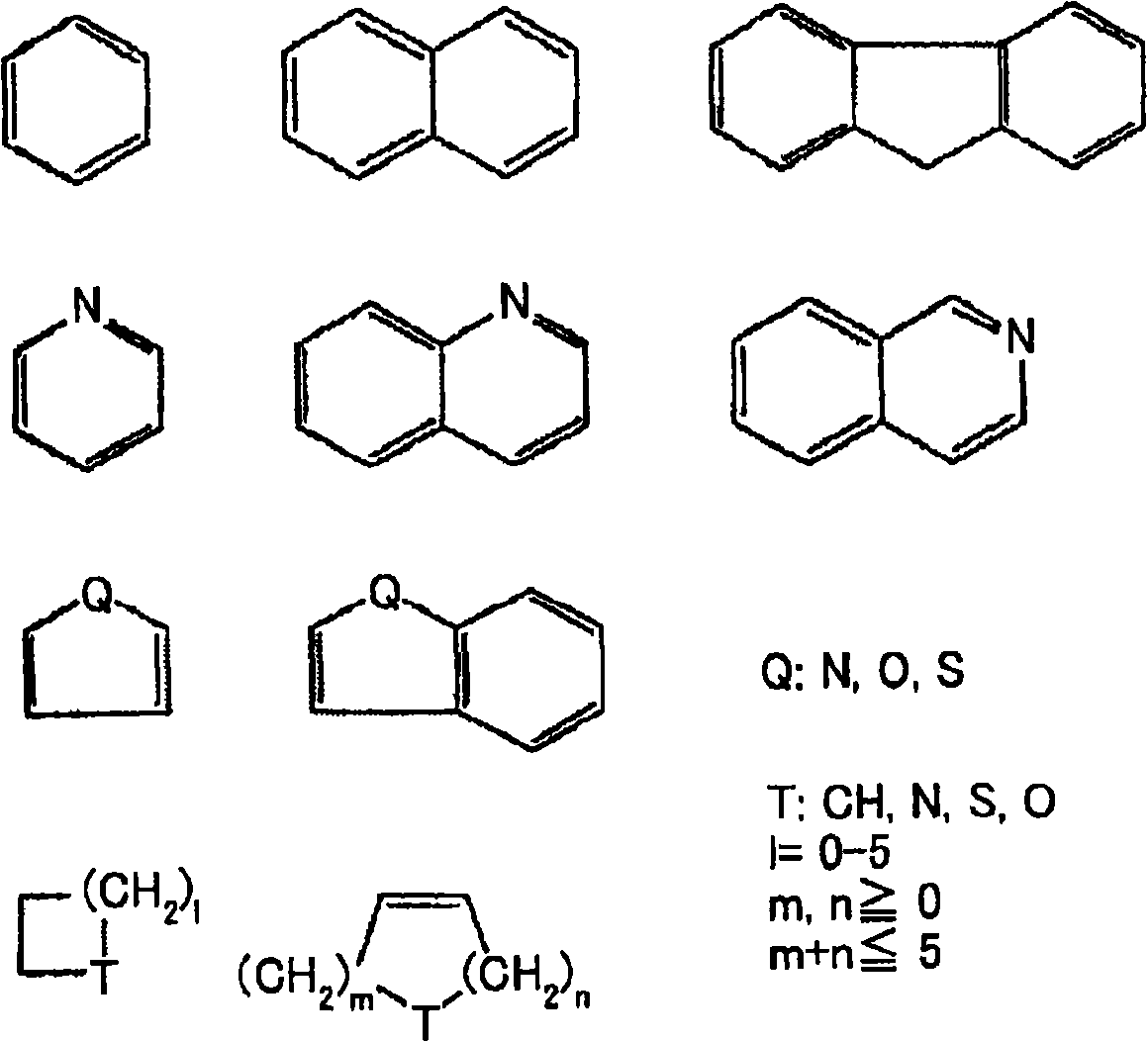
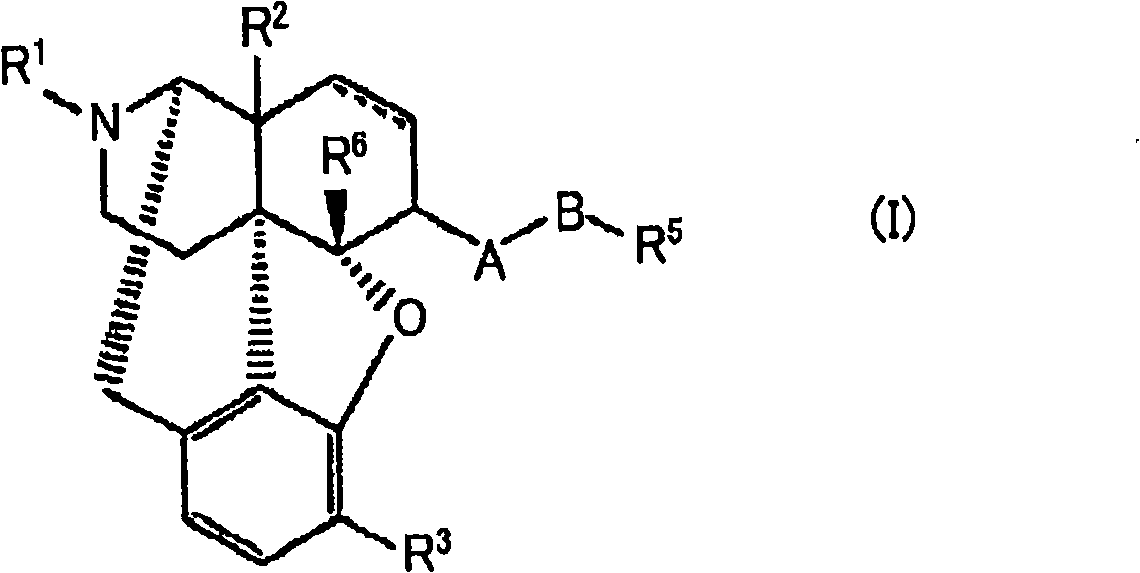
Stable solid preparation comprising 4,5-epoxymorphinan derivative
一种固体制剂、环氧基的技术,应用在含有效成分的医用配制品、非有效成分的医用配制品、丸剂输送等方向,能够解决未有硫代硫酸钠、没有稳定化效果等问题,达到保存性优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] 78.895 parts of mannitol (ペアリトル SD200, ロケツトジヤパン) was weighed, sieved through a sieve with an opening of 1 mm, and charged into a fluidized bed granulator (FLO-5, Ferrointo Industries). 0.005 parts of compound 1 and 0.1 part of sodium thiosulfate hydrate were dissolved in distilled water to obtain a spray liquid, and the obtained granulated particles (the average particle diameter measured according to the particle size determination method of the Japanese Pharmacopoeia of the 15th revision was 146 μm) Spraying is performed to prepare drug-loaded granules. 15 parts of mannitol and 5 parts of low-substituted hydroxypropyl cellulose (average particle size 50 μm, hydroxypropoxy content 10.0-12.9 wt%, loose bulk density 0.34 g / mL ( LH11, Shin-Etsu Chemical Industry) [hereinafter abbreviated as L-HPC]), mixed with a V-type mixer (permeable type S-5, Tsutsui Riken Machinery) for 15 minutes. 1 part of magnesium stearate (Taipei Chemical Industry) was further added and mixed fo...
Embodiment 2
[0086] The drug-loaded granules were prepared in the same manner as in Example 1, and 10 parts of mannitol, 10 parts of L-HPC, and 1 part of magnesium stearate were added to 79 parts of granules loaded with compound 1. Mix and tablet by the same method as Example 1.
Embodiment 3
[0088] Drug-carrying granules were prepared in the same manner as in Example 1, except that 20 parts of L-HPC and 1 part of magnesium stearate were added to 79 parts of compound 1-carried granules, and the same procedure as in Example 1 was carried out except that Method Mix and tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com