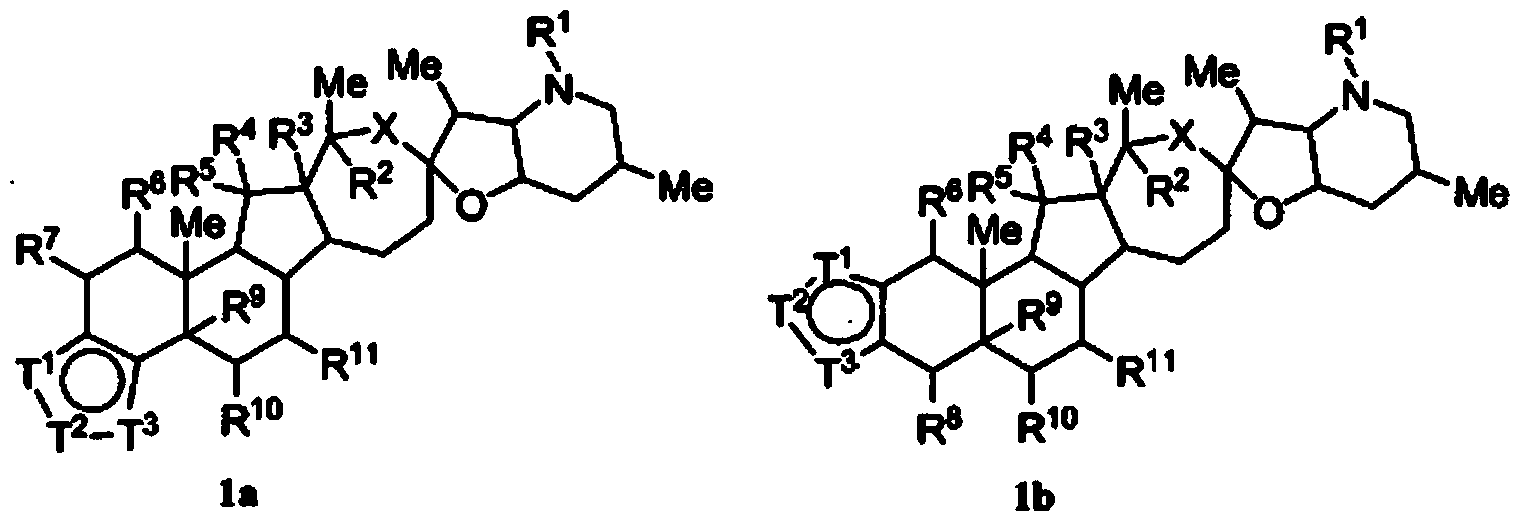
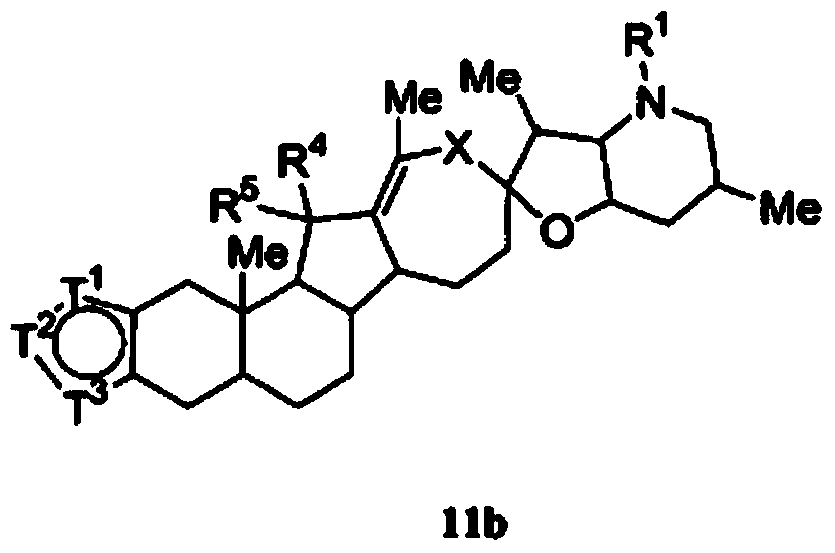
Heterocyclic cyclopamine analogs and methods of use thereof
A technology of drugs and compounds, applied in the field of cyclopamine analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
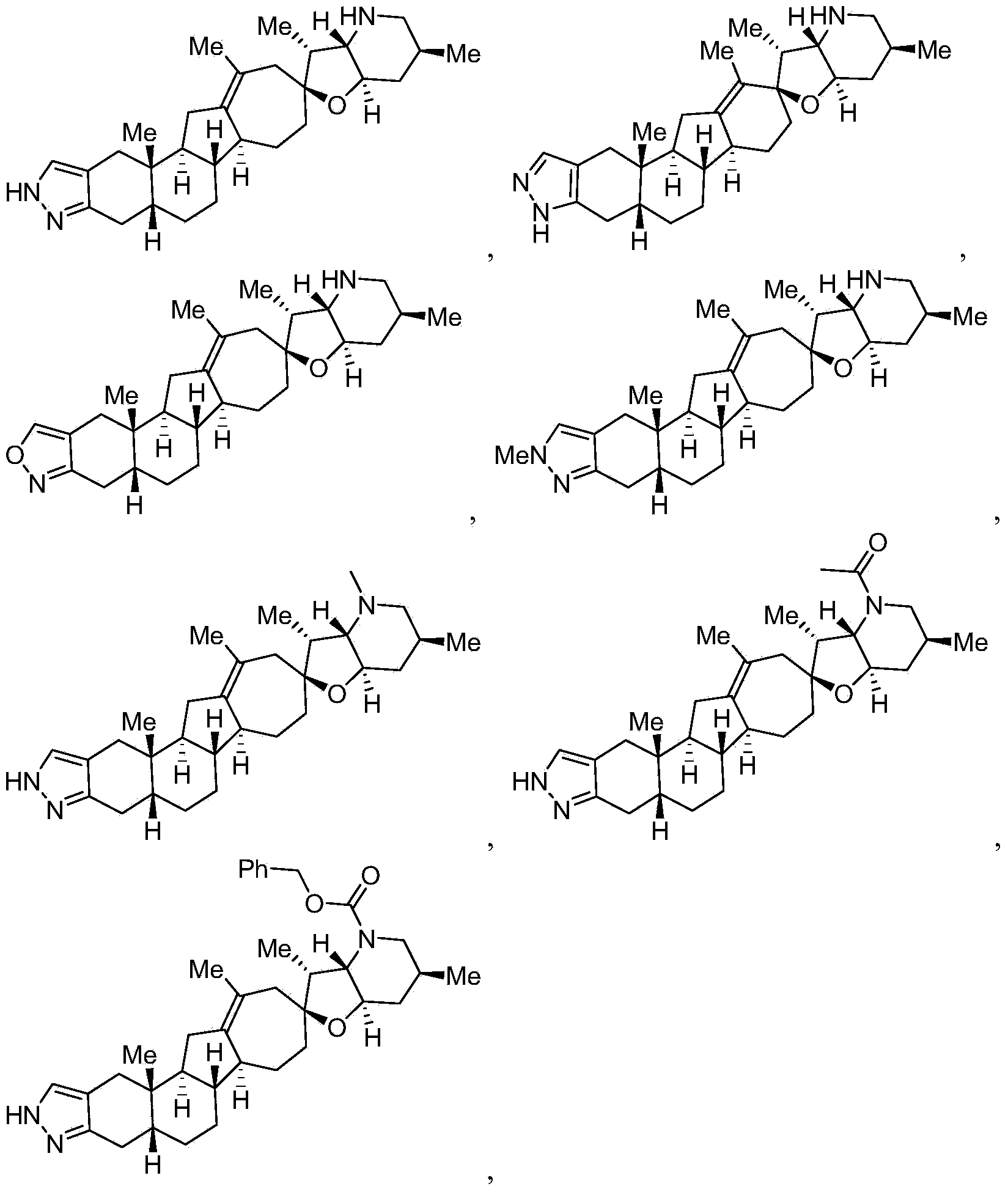
Embodiment 1
[0194]
[0195] Step A
[0196]
[0197] Cyclopamine 2 (5.02 g, 12.2 mmol, 1.0 equiv) was dissolved in anhydrous pyridine (25 mL). DMAP (300 mg, 2.44 mmol, 0.2 equiv) and triethylamine (5.5 mL, 39.1 mmol, 3.2 equiv) were added, followed by BtO-Cbz (10.5 g, 39.1 mmol, 3.2 equiv) and the mixture was heated at 40 °C for 2 h . The mixture was cooled to room temperature, treated with 30 mL of water, heated to give a homogeneous solution, and cooled to room temperature. The white precipitate formed was collected by filtration, the filter cake was washed with water (3X 50 mL) and air dried to give 9.53 g of crude product which was crystallized from toluene / heptane (1:9, 70 mL) to give 6.75 g of the desired product.
[0198] Step B:
[0199]
[0200]To a solution of diethylzinc (572 mg, 482 μL, 4.63 mmol, 3 equiv) in DCM (5.0 mL) was added bis(2,6-dimethylphenyl)phosphoric acid (1.42 g, 4.63 mmol) at -20 °C , 3 eq) in DCM (15 mL) while keeping the reaction temperature bel...
Embodiment 2
[0221]
[0222] A solution of compound 9 (100.0 mg, 0.17 mmol, 1.0 equiv) in ethanol (4 mL) was treated with hydrazine (16 mg, 0.34 mmol, 2.0 equiv) and heated at 70° C. for 0.5 h. The mixture was concentrated in vacuo and purified by flash silica gel chromatography (20→60% ether / hexanes) to give the protected pyrazole (72.0 mg) as a white solid.
[0223] In a flask equipped with a stir bar and rubber septum, the product carbamate isoxazole was dissolved in EtOAc (7ml). The solution was bubbled with nitrogen and 10% Pd / C (wet, Degussa type E101, Aldrich, 25 mg) was added. The mixture was bubbled with nitrogen, then hydrogen and stirred at room temperature for 2 hours. The mixture was then bubbled with nitrogen, filtered through a 0.45 μm polyethylene membrane, and concentrated to a clear oil. The oil was purified by flash chromatography on silica gel (0.5% ammonium hydroxide / 2→10% MeOH / DCM), concentration of the pure fractions gave an oil, lyophilization from 7% water / ter...
Embodiment 3
[0225]
[0226] Compound 11 was synthesized according to the method described in Example 2, using methylhydrazine instead of hydrazine. ([M+H] = 464.7 m / z).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com