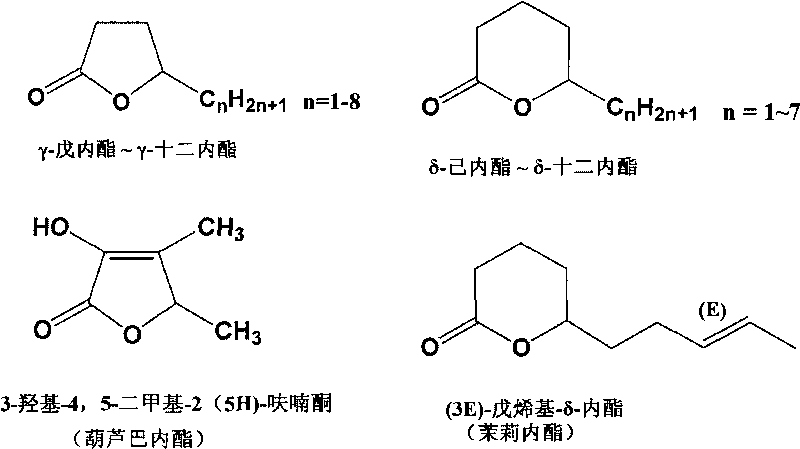
Preparation method of optical pure gamma/delta-lactone spice
A lactone and fragrance technology, which is applied in the field of optically pure γ-lactone fragrance preparation, can solve the problems of many operation steps, long time, low yield, etc., and achieve the effect of good authenticity and pure aroma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The chromatographic column specification is 250mm×10mm, the particle size is 10μm, the stationary phase filled is amylose-tris(3,5-dimethylphenylcarbamate), the column temperature is 31°C, the column pressure is 11MPa, the modifier The content of isopropanol is 2.0% v / v, the flow rate of the mobile phase is 9.4ml / min, and the wavelength of the ultraviolet detector is 220nm. 16 mg of racemic γ-nonanolide was injected for each separation, and the injection was repeated 10 times. According to the retention time of S-(-)-γ-nonanolide being 7.5 min and the retention time of R-(+)-γ-nonanolide being 8.8 min, the corresponding product fractions were collected. The solvent was removed by heating in a water bath to obtain 77 mg of S-(-)-γ-nonanolide, with an enantiomeric excess of 99%, and 78 mg of R-(+)-γ-nonanolactone, with an enantiomeric excess of 100%.
Embodiment 2
[0019] The chromatographic column specification is 250mm×10mm, the particle size is 5μm, the stationary phase filled is amylose-tris(3,5-dimethylphenylcarbamate), the column temperature is 30°C, the column pressure is 13MPa, the modifier The content of isopropanol is 2.1% v / v, the flow rate of the mobile phase is 9.2ml / min, and the wavelength of the ultraviolet detector is 220nm. 15 mg of racemic γ-undecalactone was injected for each separation, and the injection was repeated 8 times. According to the retention time of S-(-)-γ-undecalactone being 6.5 min and the retention time of R-(+)-γ-undecalactone being 7.4 min, the corresponding product fractions were collected. The solvent was removed by heating in a water bath to obtain 56 mg of S-(-)-γ-undecalactone with an enantiomeric excess of 98%, and 58 mg of R-(+)-γ-undecalactone with an enantiomeric excess of 99%.
Embodiment 3
[0021] The chromatographic column specification is 250mm×10mm, the particle size is 10μm, the stationary phase filled is cellulose-tris(benzoate), the column temperature is 31°C, the column pressure is 10MPa, the modifier isopropanol content is 1.1% v / v, and the flow The phase flow rate is 7.0ml / min, and the wavelength of the ultraviolet detector is 235nm. 15.5 mg of racemic δ-octylactone was injected for each separation, and the injection was repeated 20 times. According to the retention time of S-(-)-δ-octyl lactone being 9.5 min and the retention time of R-(+)-δ-octyl lactone being 11.2 min, the corresponding product fractions were collected. The solvent was removed by heating in a water bath to obtain 146 mg of S-(-)-δ-octylactone, with an enantiomeric excess of 99%, and 148 mg of R-(+)-δ-octylactone, with an enantiomeric excess of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com