Synthesis method of menthyl amide cooling agent
A synthetic method, the technology of menthyl amide, applied in the preparation of carboxylic acid amide, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of three wastes, complex and cumbersome processes, difficult to complete the reaction, etc., and achieve safe operation and simple process , long-lasting cooling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
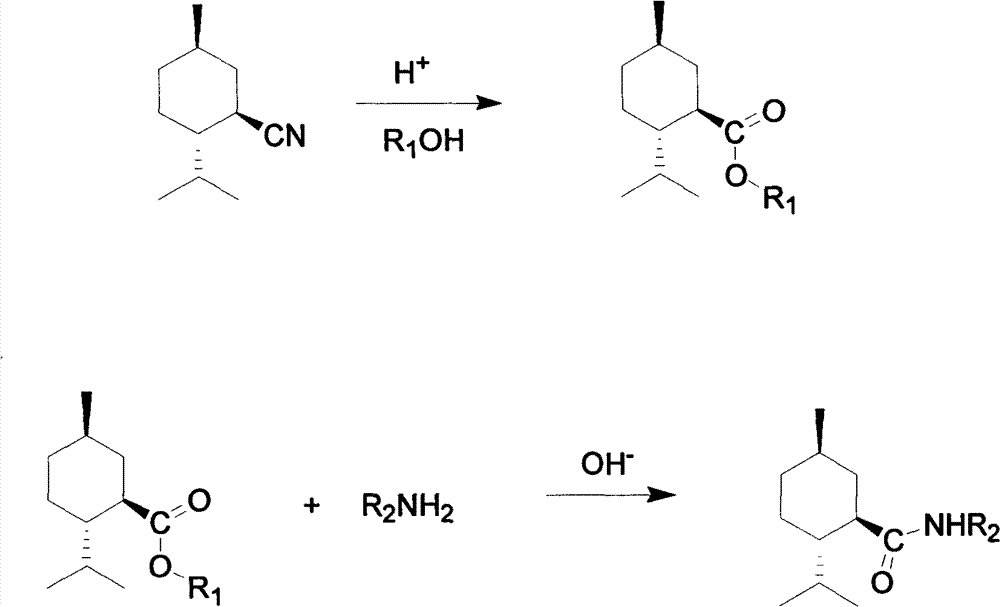
[0019] Example 1 , Synthesis of L-menthyl formate
[0020] Add 1mol L-menthaformonitrile, 2mol methanol and 1mol concentrated hydrochloric acid into a four-necked flask equipped with a thermometer and a reflux condenser, reflux under stirring conditions, and react for 2 hours;
[0021] Cool, add saturated sodium bicarbonate to neutrality, recover alcohol, cool, wash with water, stand to separate layers, dry and collect the organic layer to obtain L-menthyl formate.
Embodiment 2
[0022] The synthesis of embodiment 2, L-menthyl formate
[0023] Add 1mol L-menthacarbonitrile, 2mol methanol and 1mol sulfuric acid into a four-necked flask equipped with a thermometer and a reflux condenser, reflux under stirring conditions, and react for 2 hours;
[0024] Cool, add saturated sodium bicarbonate to neutrality, recover alcohol, cool, wash with water, stand to separate layers, dry and collect the organic layer to obtain L-menthyl formate.
Embodiment 3
[0025] Example 3 , Synthesis of L-menthyl formate
[0026] Add 1mol L-menthaformonitrile, 3mol methanol and 2mol phosphoric acid into a four-neck flask equipped with a thermometer and a reflux condenser, reflux under stirring conditions, and react for 4 hours;
[0027] Cool, add saturated sodium bicarbonate to neutrality, recover alcohol, cool, wash with water, stand to separate layers, dry and collect the organic layer to obtain L-menthyl formate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com