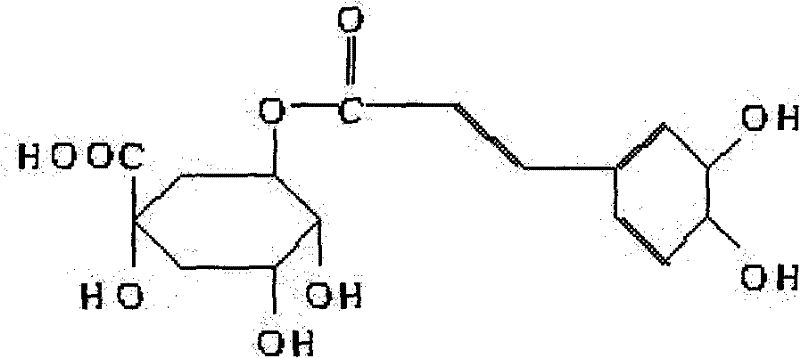
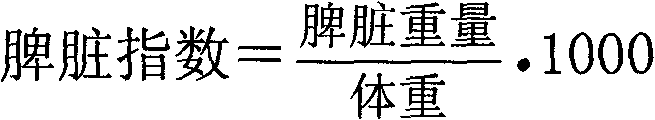Use of chlorogenic acid in preparing medicament for treating marrow fibrillation
A technology for bone marrow fibrosis and chlorogenic acid, which can be used in drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., and can solve the problems that chlorogenic acid has not yet been discovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0035] [Experimental example 1] Effect of chlorogenic acid on bone marrow suppression caused by physical factors in mice
[0036] Chlorogenic acid, with a content of 99.56%, is formulated into the required concentration with sodium chloride injection. Positive control drug: Recombinant human granulocyte colony stimulating factor injection, 300ug / tube, 1.2ml / tube.
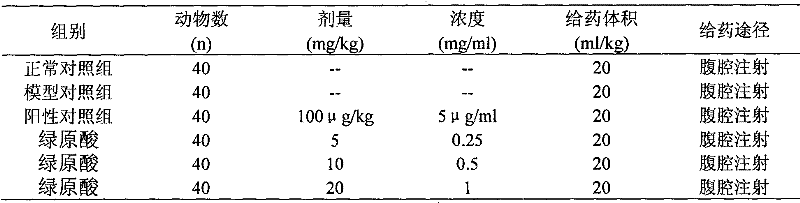
[0037] SPF ICR mice weighing 22-30g, 240 mice. According to body weight and sex, they were randomly divided into high, medium and low-dose chlorogenic acid groups, positive control group, model control group and normal control group, with 40 animals in each group, half male and female. All animals except the normal control group are used 60 Whole body irradiation of Coγ-rays, the irradiation dose is 4Gy (dose rate is 256Gy / h, irradiation time is 2min), after irradiation, each group is given corresponding test substance according to Table 1 (control group and model group are given volume of sodium chloride injection ), ...
experiment example 2
[0094] [Experimental example 2] The effect of chlorogenic acid on bone marrow suppression caused by chemical factors in dogs
[0095] Chlorogenic acid, content 99.56%, positive control drug: Li Kejun tablets, 20 mg / tablet, three times a day (3 tablets), cyclophosphamide for injection (abbreviated as CY), white powder, 200 mg / ampoule, 5 bottles / Boxed. 36 Beagle dogs (male and female), normal, healthy, uniform in weight, female not pregnant, weighing 6-7kg, and age 6 months.
[0096] The dosage design is shown in Table 19.
[0097] Table 19 Dose Design
[0098]
[0099] In addition to the normal control group, dogs in the other 5 groups were intravenously injected with 8 mg / ml cyclophosphamide 0.8 ml / kg (8 mg / kg) once a day for 5 consecutive days. On the 6th day, each test drug was given according to Table 19. The model control group and the normal control group were given normal saline for 13 consecutive days. Before modeling, every day after modeling, and 2, 4, 6, 8, 10, 12, and 1...
experiment example 3
[0127] [Experimental example 3] Chlorogenic acid pair 60 Protective effects of Co-γ rays on the damage of hematopoietic stem cells in spleen of mice
[0128] 1. Experimental method
[0129] Take Health C 57 60 mice were randomly divided into 5 groups according to gender and body weight. The rats in the negative group were given intraperitoneal injection of 0.4ml / 20g body weight with normal saline, and the mice in the positive group (granulocyte colony stimulating factor) were given intraperitoneal injection of 0.4ml / 20g body weight (2ug / kg). 0.1%, 0.05% and 0.025% of chlorogenic acid liquid was injected intraperitoneally with 0.4ml / 20g body weight (dose 20, 10 and 5mg / kg) to each mouse in each group. The rats in the above 5 groups were administered once a day for 7 consecutive days. On the 7th day, the surviving animals were weighed, 1 hour after the last administration. The mice in each group were sacrificed by cervical dislocation on the 9th day after whole body irradiation. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com