Pharmaceutical composition of dsRNA and saikosaponin and application thereof
A technology of saikosaponin and bupleurum, which is applied in the drug combination of dsRNA and saikosaponin and its application field, which can solve the problems of no compatibility of active substances, lack of suitable conditions for double-strand pairing, systematic research on factors affecting stability, low fever And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0016] Embodiment 1: This embodiment adopts the following technical scheme: its formula consists of: polykinocytes, saikosaponin and Bupleurum volatile oil; its preparation method is: adding appropriate pharmaceutical excipients to the above components by a certain amount The process is made into a compound preparation; these components have certain synergistic and synergistic effects, and can avoid the side effects of dsRNA.
specific Embodiment approach 2
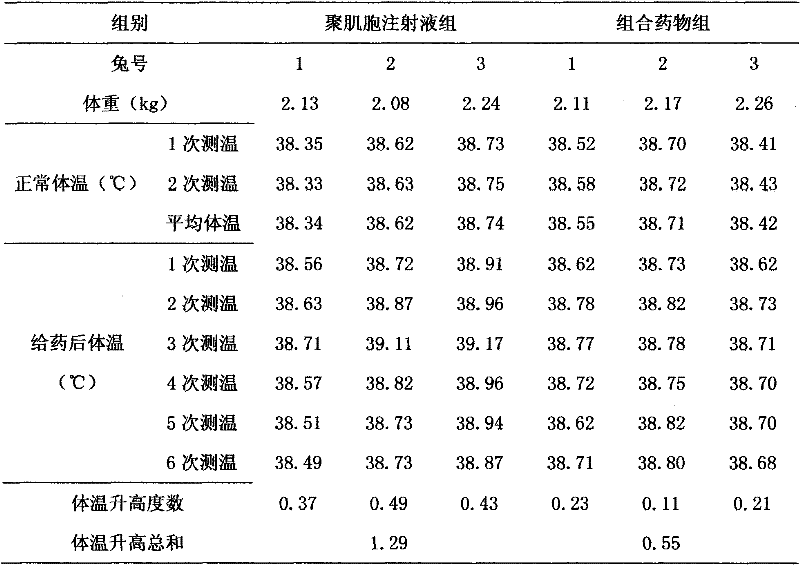
[0017] Specific implementation mode two: refer to figure 1 , The specific embodiment adopts the following technical scheme: take two groups of test rabbits, inject the simple polyinocide injection and the combined medicine respectively to carry out the pyrogen test, and record the temperature changes of the test rabbits. The results showed that the combined drug could alleviate the pyrogen reaction of poly-inosin injection alone.
specific Embodiment approach 3
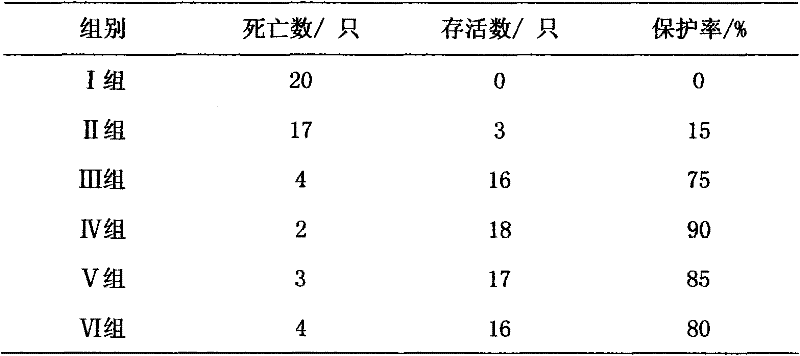
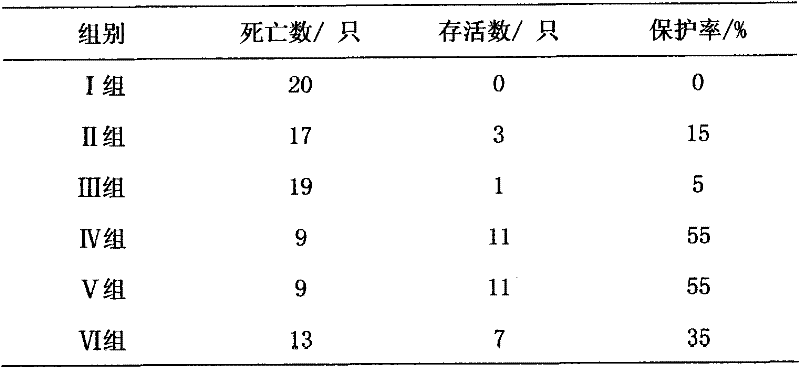
[0018] Specific implementation mode three: refer to figure 2 with image 3 , This specific embodiment adopts the following technical scheme: the medicine is diluted to 50 μg / ml with water for injection. 240 test chicks were randomly divided into 6 test groups, 40 in each group. Group I: blank control group; Group II: drug control group (astragalus polysaccharide injection, 20 mg per kilogram of body weight); Group III: vaccine control group (no drug); Group IV: high-dose drug test group (0.1 mg per kilogram of body weight). mg); V group: middle dose drug test group (0.05 mg per kg body weight); VI group: low dose drug test group (0.01 mg per kg body weight). The above chicks were reared in isolated cages, and the blank control group, the positive control group and the medication test group were raised in different chicken houses, and the feeding conditions were kept consistent. After feeding for 10 days, four groups III, IV, V and VI were immunized with Newcastle disease v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com