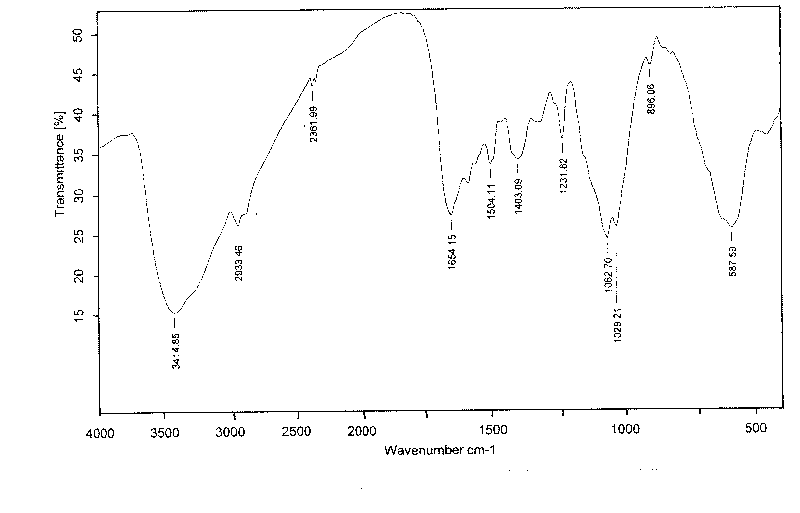
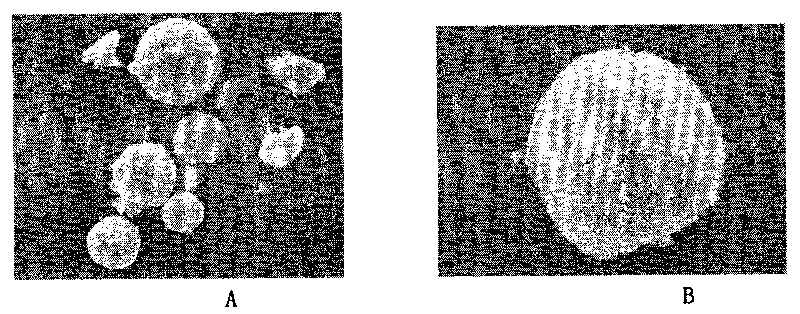
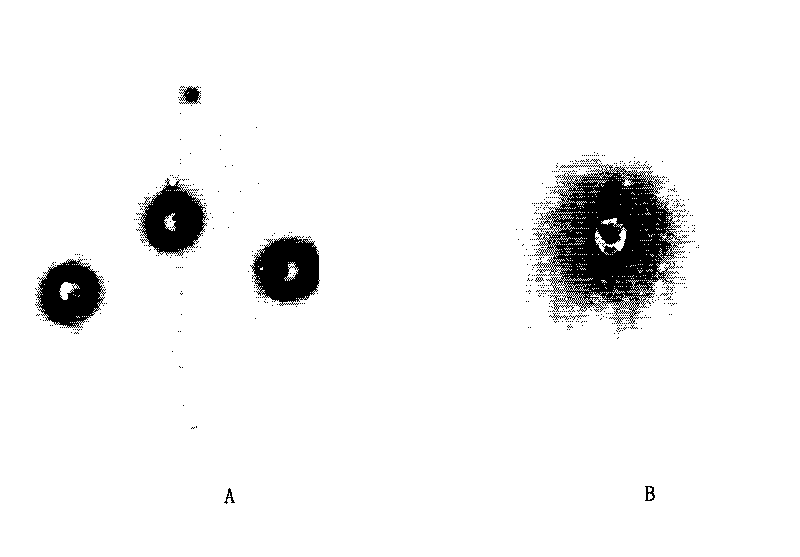
Vancomycin slow release microsphere with magnetic filed compliance and preparation method thereof
A technology of vancomycin, slow-release microspheres, applied in the field of chemistry, to achieve the effect of high local drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of vancomycin sustained-release microspheres with magnetic field compliance
[0050] (1) preparation of chitosan acetic acid solution
[0051] 1g chitosan is dissolved in 1% acetic acid solution 100ml, is made into the chitosan acetic acid solution of 1% (g / g) concentration;
[0052] (2) Preparation of aqueous phase liquid
[0053] Nanoscale iron ferric oxide 0.1g and vancomycin 0.1g are dissolved in the chitosan acetic acid solution that 20ml step (1) makes to obtain aqueous phase liquid;
[0054] (3) Preparation of oil phase liquid
[0055] Add 80ml of ethyl benzoate to 1ml of Span-80 to obtain an oil phase liquid;
[0056] (4) Preparation of emulsion
[0057] Add the water phase liquid prepared in step (2) into the oil phase prepared in step (3) for emulsification to obtain an emulsified liquid. The emulsification conditions are: temperature 20°C, stirring speed 500 rpm, emulsification time 20 min;
[0058] (5) Formation of microspheres
[0...
Embodiment 2
[0062] Example 2 Preparation of vancomycin sustained-release microspheres with magnetic field compliance
[0063] (1) preparation of chitosan acetic acid solution
[0064] 1g chitosan is dissolved in 2% acetic acid solution 100ml, is made into the chitosan acetic acid solution of 1% (g / g) concentration;
[0065] (2) Preparation of aqueous phase liquid
[0066] Nanoscale iron ferric oxide 0.2g and vancomycin 0.1g are dissolved in the chitosan acetic acid solution that 20ml step (1) makes to obtain aqueous phase liquid;
[0067] (3) Preparation of oil phase liquid
[0068] Add 100ml of ethyl benzoate to 2ml of Span-80 to obtain an oil phase liquid;
[0069] (4) Preparation of emulsion
[0070] Add the water phase liquid prepared in step (2) into the oil phase prepared in step (3) to emulsify to obtain an emulsified liquid. The emulsification conditions are: temperature 30°C, stirring speed 1000rpm, emulsification time 30min;
[0071] (5) Formation of microspheres
[0072] ...
Embodiment 3
[0075] Example 3 Preparation of vancomycin sustained-release microspheres with magnetic field compliance
[0076] (1) preparation of chitosan acetic acid solution
[0077] 1g chitosan is dissolved in 2% acetic acid solution 100ml, is made into the chitosan acetic acid solution of 1% (g / g) concentration;
[0078] (2) Preparation of aqueous phase liquid
[0079] Nanoscale iron ferric oxide 0.2g and vancomycin 0.2g are dissolved in the chitosan acetic acid solution that 20ml step (1) makes to obtain aqueous phase liquid;
[0080] (3) Preparation of oil phase liquid
[0081] Add 60ml of ethyl benzoate to 2ml of Span 80 to obtain an oil phase liquid;
[0082] (4) Preparation of emulsion
[0083] Add the water phase liquid prepared in step (2) into the oil phase prepared in step (3) to emulsify to obtain an emulsified liquid. The emulsification conditions are: temperature 40°C, stirring speed 1500rpm, emulsification time 30min;
[0084] (5) Formation of microspheres
[0085] A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com