Anti-angiogenic hydrogel sustained-release preparation and application thereof
An anti-angiogenesis and sustained-release preparation technology, applied in the field of hydrogel sustained-release preparations, can solve the problems of amplifying systemic toxicity, affecting the normal cardiovascular system, etc., so as to reduce the number of administrations, avoid toxicity, and reduce systemic side effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
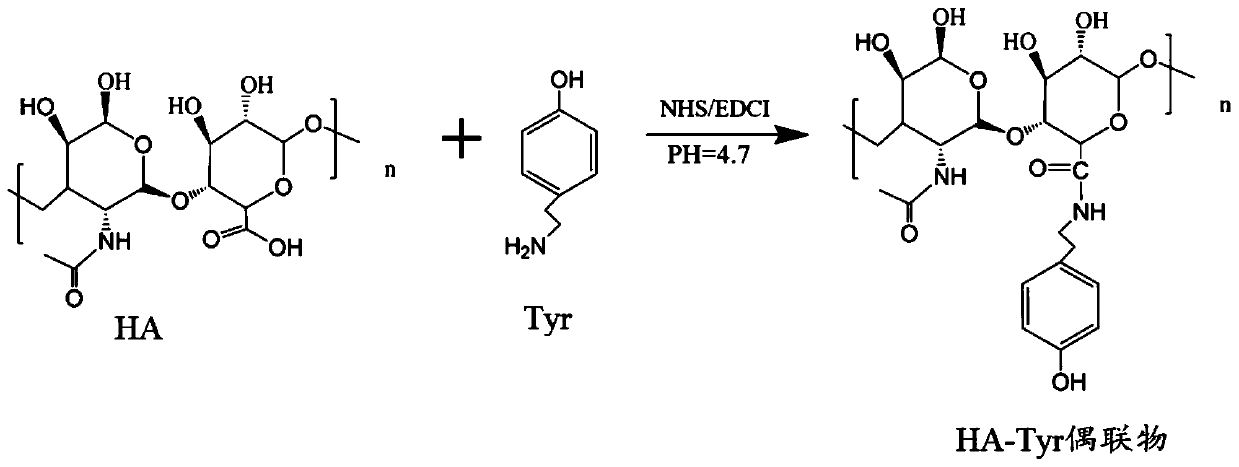
[0022] Example 1: Preparation of HA-Tyr conjugates
[0023] Weigh 1 g of HA (hyaluronic acid) and 210 mg of Tyr (tyrosine) with a number average molecular weight of about 38,000 to 90,000 and place them in a sterile glass bottle, add 100 mL of deionized water, and place the beaker on a magnetic stirrer to Stir and dissolve at a rate of 800r / min. After complete dissolution, continue to add 1.2g NHS and 1.95g EDAC·HCl, and continue to stir and react at room temperature for 24h. The reaction process is as follows: figure 1 shown. After the reaction, adjust the pH of the reaction solution to be 5 to 7, and pour the reaction solution into a dialysis bag with a molecular weight cut-off of 3500Da. ) for 1 day each, and then freeze-dry the dialyzed solution to obtain a white floc, namely the HA-Tyr conjugate, which was sterilized and stored at low temperature for later use. In addition, different types of HA-Tyr conjugates were prepared by using HA with different number-average mole...
Embodiment 2
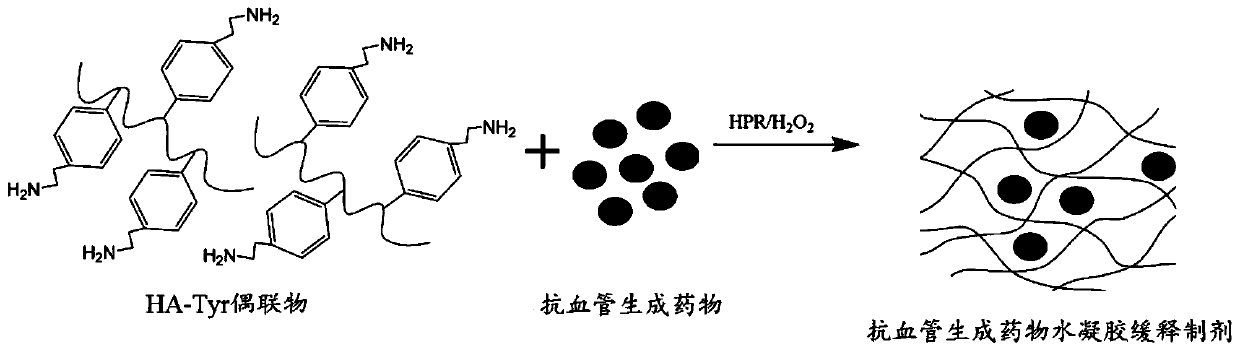
[0028] Example 2: Preparation of an anti-angiogenic hydrogel sustained-release preparation
[0029] Weigh the HA-Tyr conjugate prepared by using the ratio No. 1 in Table 1, and prepare multiple solutions with concentration gradients. The concentrations of these solutions are in the range of 10mg / mL~20mg / mL, and then the The angiogenesis drugs were formulated into multiple solutions with concentration gradients, and the concentrations of these solutions were in the range of 0.05 mg / mL to 25 mg / mL, and the HPR solution with a concentration of 25 U / mL and hydrogen peroxide with a concentration of 800 μM were taken for use. Prepare hydrogel slow-release preparation according to the proportioning in table 2, the formation process of slow-release preparation is as follows figure 2 shown.
[0030] Table 2 The proportioning relationship of the hydrogel sustained-release preparation
[0031]
Embodiment 3
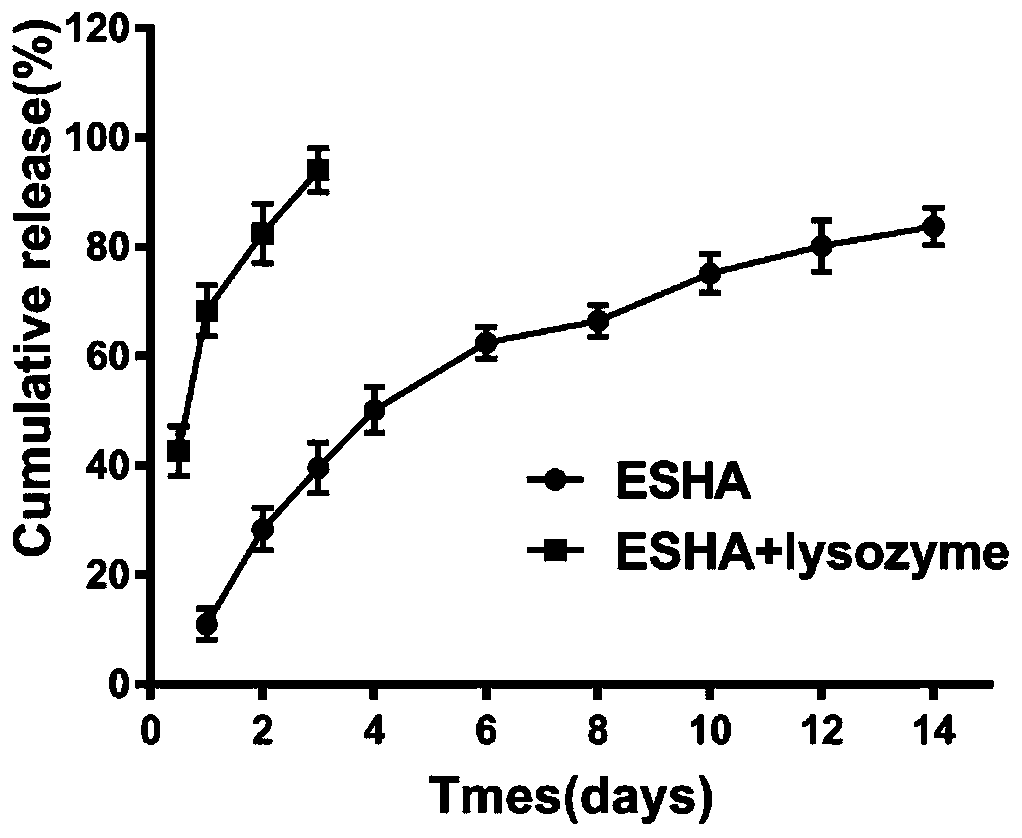
[0032] Example Three: In Vitro Drug Release of Hydrogel Sustained Release Preparation
[0033] After mixing the materials according to the proportioning relationship in Table 2, a hydrogel will be formed in about 50s. The in vitro drug release rate of No. 1 proportioning relationship was investigated, and the Endostar water added with hyaluronidase was also investigated. The in vitro drug release rate of the gel, the results are as follows image 3 shown. It can be seen that the anti-angiogenic hydrogel sustained-release preparation of the present invention shows the characteristics of sustained slow release, and it releases about 80% on the 14th day, and the addition of 20~30U / mL hyaluronidase anti- The angiogenic hydrogel drug release was close to 100% on day 3. The hydrogel sustained-release preparations formulated according to the proportioning relationship of No. 2 and No. 3 in Table 2 also showed the characteristics of sustained and slow release.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com