Method for compounding allyl amyl glycolate
A synthesis method and the technology of Gapon ester are applied in the field of preparation of daily chemical fragrances, which can solve the problems of complicated process and complicated raw materials, and achieve the effects of environmental safety and friendliness, easy operation and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
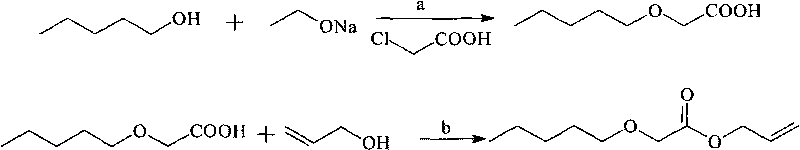
[0027] The first step, the preparation of isoamyloxyacetic acid
[0028] In a 500 ml three-necked flask equipped with a stirrer, a thermometer, and a fractionating column, add 15 grams (0.22 mol) of sodium ethoxide and 290 grams of isoamyl alcohol (3.3 mol), stir, start heating, and slowly distill the alcohol to the top. Warm at 125°C to obtain sodium isoamyloxide solution. Cool to an internal temperature of 60-80°C, start adding 0.25 mol of chloroacetic acid, and continue to react for 2 hours at an internal temperature of 65°C. Then, it was heated to an internal temperature of 130° C. to recover isoamyl alcohol. Finally, 200 ml of water was added and steam distillation was performed to remove residual isoamyl alcohol. After the reaction solution was cooled, add 20% hydrochloric acid to acidify, separate the organic layer, wash with water, dry, and collect the fraction at 116-118°C / 5mmHg by distillation under reduced pressure to obtain 13.9 grams of isopentyloxyacetic acid (...
Embodiment 2
[0032] The first step, the preparation of isoamyloxyacetic acid
[0033] In a 500 ml three-necked flask equipped with a stirrer, a thermometer, and a fractionating column, add 15 grams (0.22 mol) of sodium ethoxide and 290 grams of isoamyl alcohol (3.3 mol), stir, start heating, and slowly distill the alcohol to the top. Warm at 125°C to obtain sodium isoamyloxide solution. Cool to an internal temperature of 65°C, add 9.5 g (0.1 mol) of chloroacetic acid, and continue the reaction at an internal temperature of 60°C for 2 hours. Then, it is heated to an internal temperature of 135° C. to recover isoamyl alcohol. Finally, 200 ml of water was added and steam distillation was performed to remove residual isoamyl alcohol. After the reaction solution was cooled, add 52.8 grams of 20% hydrochloric acid to acidify it, separate the organic layer, wash with water and dry it, and collect the fraction at 116-118°C / 5mmHg by distillation under reduced pressure to obtain 13.8 grams (95.4%)...
Embodiment approach 3
[0037] The first step, the preparation of isoamyloxyacetic acid
[0038] In a 500 ml three-necked flask equipped with a stirrer, a thermometer, and a fractionating column, add 15 grams (0.22 mol) of sodium ethoxide and 290 grams of isoamyl alcohol (3.3 mol), stir, start heating, and slowly distill the alcohol to the top. Warm at 125°C to obtain sodium isoamyloxide solution. Cool to an internal temperature of 60°C, add 18.9 g (0.2 mol) of chloroacetic acid, and continue the reaction at an internal temperature of 60°C for 1 hour. Then, it was heated to an internal temperature of 130° C. to recover isoamyl alcohol. Finally, 200 ml of water was added and steam distillation was performed to remove residual isoamyl alcohol. After the reaction solution was cooled, add 105.6 grams of 20% hydrochloric acid to acidify it, separate the organic layer, wash with water and dry it, then collect the fraction at 116-118°C / 5mmHg by distillation under reduced pressure to obtain 25.9 grams (96....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com