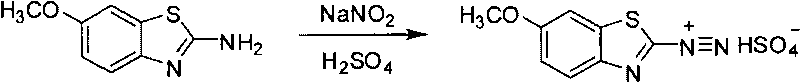Preparation method of environment-friendly zinc-free cationic dye
A zinc cation, environment-friendly technology, applied in azo dyes, organic dyes, onium-based azo dyes, etc., can solve the problems of excessive zinc concentration, harsh dye production environment, and increased raw material costs, and achieve low zinc content in products , the production process is environmentally friendly, and the effect of industrial application prospects is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
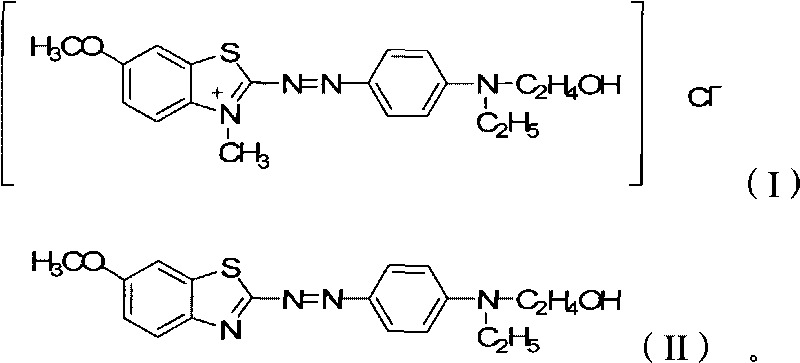
[0028] 35.6 kilograms (0.1mol) that have been converted into raw material A shown in formula (II) are stirred with 534 kilograms of water in the enamel reactor, add 8.1 kilograms (0.2mol) of magnesium oxide that have been reduced and continue stirring, heating and Keep the temperature at 35°C, slowly add 19.3 kilograms (0.15mol) of 98% dimethyl sulfate, keep warm and stir for 5 hours after adding, monitor the reaction solution with TLC without Compound A as the end of the reaction, slowly add 160.2 kilograms of chlorine Sodium chloride, stirred for 6 hours, filtered, and the filter cake was dried to obtain 39.9 kg of environmentally friendly zinc-free cationic dyes, with a molar yield of 98.0%, moisture ≤ 7%, and zinc content ≤ 50ppm.
Embodiment 2
[0030] The preparation process is substantially the same as the method of Example 1. The difference from Example 1 is that the amount of solvent water added is 712 kilograms, and the amount of sodium chloride added is 213.6 kilograms to obtain 38.7 kilograms of environmentally friendly zinc-free cationic dyes. Yield 95.1%, moisture ≤ 7%, zinc content ≤ 40ppm.
Embodiment 3
[0032] The preparation process is substantially the same as the method of Example 1. The difference with Example 1 is that the addition of solvent water is 1068 kilograms, and the amount of sodium chloride added is 284.8 kilograms to obtain 36.6 kilograms of environmentally friendly zinc-free cationic dyes. Yield 89.9%, moisture ≤ 7%, zinc content ≤ 30ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com