Artemisine solid lipid nano-particles and preparing method thereof
A technology of solid lipid nanometer and artemisinin, which is applied in the field of new pharmaceutical dosage forms and their preparation, can solve the problems of low biological potency, only bioavailability, and allergic reactions of patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
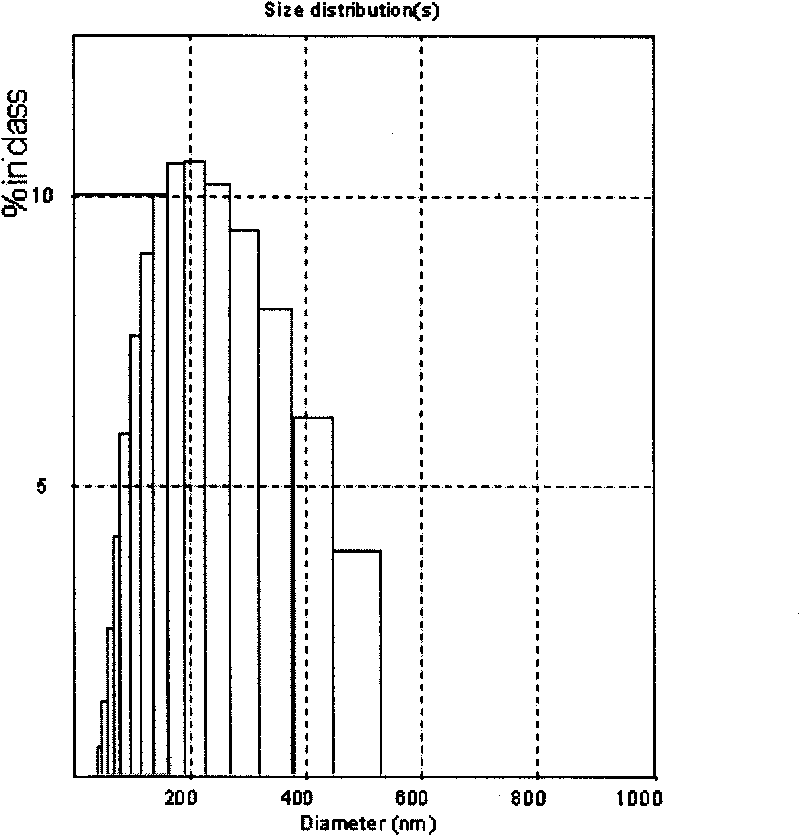
[0077] Weigh 80 g of glyceryl monostearate and place it in a water bath at 75°C until glyceryl monostearate melts. Weigh 5 g of artemisinin, and dissolve artemisinin in glycerol monostearate oil phase. In addition, 8 g of Poloxamer 407 was weighed as an emulsifier and dissolved in water at the same temperature. Mix the oil phase dissolved in artemisinin with the water phase, heat and stir at 500rpm for 20 minutes to form a coarse milk; use a high-pressure milk homogenizer to homogenize the coarse milk 10 times to obtain an ultra-fine emulsion, and quickly cool the ultra-fine emulsion to 2°C to obtain artemisinin solid lipid nanoparticles. The average particle size is about 210nm, see the particle size distribution diagram figure 1 , it can be seen from the figure that the particle size distribution is very narrow.
Embodiment 2
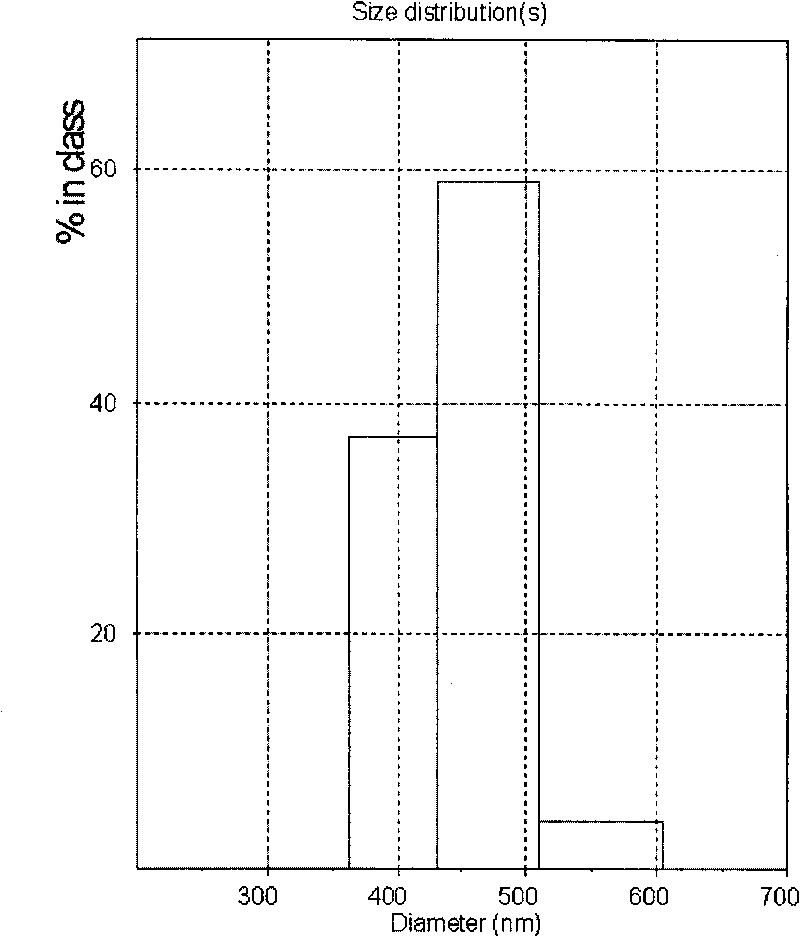
[0079] Weigh 100g of stearic acid, 5g of artemisinin, and 2g of soybean lecithin for injection, put stearic acid and soybean lecithin into a water bath at 73°C until stearic acid and soybean lecithin melt, and then add artemisinin Add and stir to dissolve the artemisinin in the molten stearic acid to form an oil phase. In addition, weigh about 20g of poloxamer F68, dissolve it in water at the same temperature, then mix the oil phase with the water phase, keep the temperature, and stir at 800rpm for about 30 minutes to form a rough milk; ultrasonically treat the thick milk for 10 minutes , and then rapidly cooled to 3°C to obtain artemisinin solid lipid nanoparticles. The average particle size is about 443.5nm, see the particle size distribution diagram figure 2 , it can be seen from the figure that the particle size distribution is very narrow.
Embodiment 3
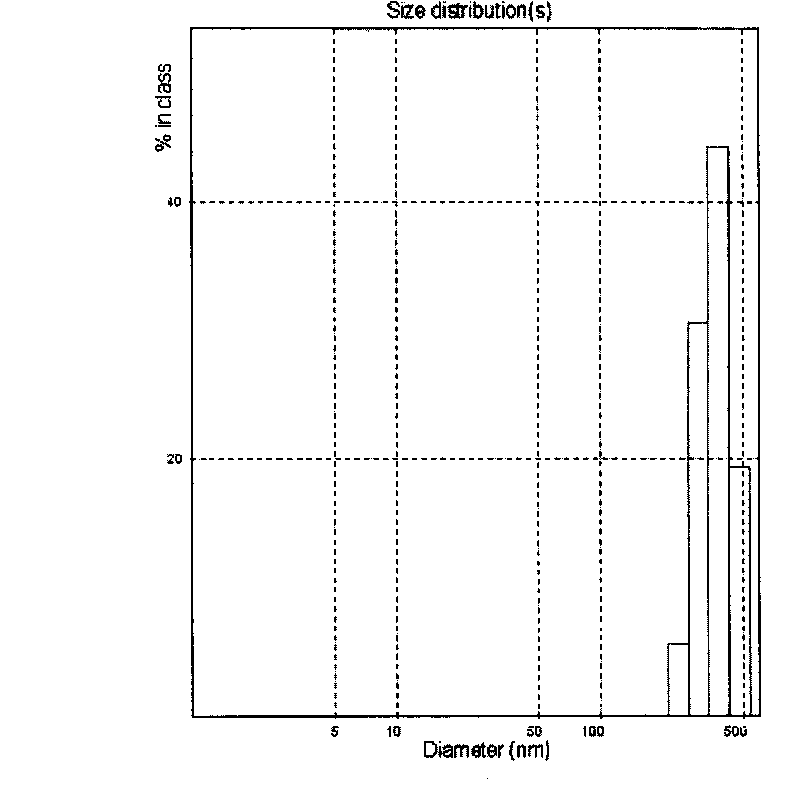
[0081] Weigh 80 g of glyceryl monostearate and place it in a water bath at 75±5°C until glyceryl monostearate melts. Weigh 5.6 g of artemisinin, and dissolve artemisinin in glycerol monostearate oil phase. In addition, 8 g of Poloxamer 407 was weighed as an emulsifier and dissolved in water at the same temperature. Mix the oil phase in which artemisinin is dissolved with the water phase, heat and stir at 500rpm for 20 minutes to form a rough milk; use a high-speed stirrer to process the thick milk at 10000rpm for 10 minutes, and then use ultrasonic treatment for 10 minutes to obtain Ultrafine emulsion, cooling the ultrafine emulsion to 2°C rapidly to obtain artemisinin solid lipid nanoparticles. The average particle size is about 367.7nm, see the particle size distribution diagram image 3 , it can be seen from the figure that the particle size distribution is very narrow.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com