Kit for real-time fluorescence RT-PCR detection of H9 subtype avian influenza virus
A bird flu virus and kit technology, applied in the field of real-time fluorescent RT-PCR test kits for H9 subtype bird flu virus, can solve the problems of poor sensitivity and specificity, can not accurately reflect whether it is infected or carries the virus, and achieves effective curative effect The effect of monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Development of one-step real-time fluorescent RT-PCR detection reagent for H9 subtype avian influenza virus
[0030] 1. Design of primers and probes: by comparing and analyzing the existing AIV-H9 nucleic acid sequences in the Genbank database and the nucleic acid sequences reported in domestic and foreign published documents, the matrix protein-encoding gene (M The conserved fragment of gene) is the amplification target site, select a highly conserved segment with no secondary structure, and use software to manually design multiple pairs of primers and probes according to the basic principles of primer and probe design.
[0031] 2. Selection of samples: According to relevant domestic and foreign literature reports, throat swabs, cloacal swabs, muscle or organ samples, serum or plasma samples can be selected.
[0032] 3. Establishment and optimization of the reaction system
[0033] Sample preparation: 3 AIV-H9 samples identified as positive by virus culture...
Embodiment 2
[0043] Example 2: One-step real-time fluorescent RT-PCR detection kit for H9 subtype avian influenza virus and its use
[0044] 1. Prepare a kit including the following components: 1 tube of RNA extraction solution (50ml / tube), 24 tubes of RT-PCR amplification reaction solution (20μl / tube), 1 tube of negative quality control (100μl / tube), 1 tube of positive Quality control substance (100μl / tube) 1 tube, quantitative reference substance (50μl / tube) 4 tubes.
[0045] 2. Specimen collection, transportation and storage
[0046] 2.1 Applicable sample types: throat swabs, cloacal swabs, muscle or organ samples, serum or plasma, etc.
[0047] 2.2 Sample collection and pretreatment (note aseptic operation)
[0048] 2.2.1 Live poultry samples—take throat swabs, cloacal swabs or fresh feces swabs. The specific collection methods are as follows:
[0049] 1) Throat swab: When taking it, the swab should be deep into the throat and upper palate and scraped back and forth 3 to 5 times to ...
Embodiment 3
[0066] Example 3: Quantitative detection of clinical samples using one-step real-time fluorescent RT-PCR detection kit for H9 subtype avian influenza virus
[0067] All experimental procedures were completed in the National Avian Influenza Reference Laboratory of Harbin Veterinary Research Institute, and clinical samples were provided by the National Avian Influenza Reference Laboratory of Harbin Veterinary Research Institute, including throat swabs, cloacal swabs, muscle or organ samples and other sample types; specimens RNA extraction, RT-PCR reaction and result analysis were carried out with reference to Example 2.
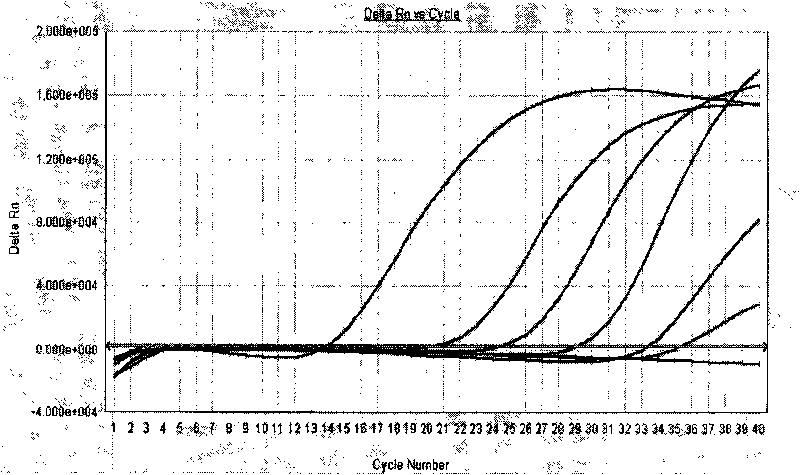
[0068] After the RT-PCR reaction is over, adjust the analysis parameters according to the amplification curve, so that the standard curve of the quantitative reference product under the standard curve (Std curve) window reaches the best (that is, the square of the correlation coefficient (R 2 )>0.97), and then analyze the clinical samples. The test results of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com